Synthesis ( IF 2.2 ) Pub Date : 2024-09-20 , DOI: 10.1055/s-0043-1775395 Zhuohan Fang, Qian Xu, Xuehe Lu, Nianfeng Wan, Wu-Lin Yang
|
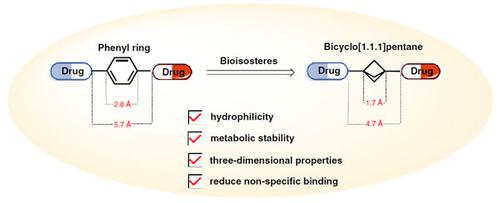
Bicyclo[1.1.1]pentane (BCP) is a bridging ring skeleton with three-dimensional structure. BCP is a bioisostere of the phenyl ring, tert-butyl group, and alkynes; it has excellent physical and chemical properties compared with phenyl ring, so it has been widely considered by the pharmaceutical chemistry. This short review examines related reports of BCP as a bioisostere of the phenyl ring, and the changes in physicochemical properties and biological activity after substitution. The solubility, clogP, and metabolic toxicity of the drug are improved by BCP bioisosteres, but the biological activity of BCP is lower than the phenyl ring. The application of BCP in drug research and development will be further expanded to provide more possibilities for future drug innovation and development.
1 Introduction
2 Replacement of Phenyl Rings by BCP
3 Conclusion
中文翻译:

双环[1.1.1]戊烷作为苯环生物等排体在药物化学中的应用
双环[1.1.1]戊烷(BCP)是具有三维结构的桥环骨架。 BCP是苯环、叔丁基和炔烃的生物等排体;与苯环相比,它具有优异的物理和化学性质,因此受到药物化学界的广泛考虑。本文综述了BCP作为苯环生物等排体的相关报道,以及取代后理化性质和生物活性的变化。 BCP生物等排体改善了药物的溶解度、clogP和代谢毒性,但BCP的生物活性低于苯环。 BCP在药物研发中的应用将进一步拓展,为未来药物创新开发提供更多可能。
1 简介
2 BCP取代苯环
3 结论


















































 京公网安备 11010802027423号
京公网安备 11010802027423号