Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of vaccines from low to high immunogenicity by antibodies with epitope complementarity
Immunity ( IF 25.5 ) Pub Date : 2024-09-20 , DOI: 10.1016/j.immuni.2024.08.017 Alexandra R. Dvorscek, Craig I. McKenzie, Vera C. Stäheli, Zhoujie Ding, Jacqueline White, Stewart A. Fabb, Leonard Lim, Kristy O’Donnell, Catherine Pitt, Daniel Christ, Danika L. Hill, Colin W. Pouton, Deborah L. Burnett, Robert Brink, Marcus J. Robinson, David M. Tarlinton, Isaak Quast
Immunity ( IF 25.5 ) Pub Date : 2024-09-20 , DOI: 10.1016/j.immuni.2024.08.017 Alexandra R. Dvorscek, Craig I. McKenzie, Vera C. Stäheli, Zhoujie Ding, Jacqueline White, Stewart A. Fabb, Leonard Lim, Kristy O’Donnell, Catherine Pitt, Daniel Christ, Danika L. Hill, Colin W. Pouton, Deborah L. Burnett, Robert Brink, Marcus J. Robinson, David M. Tarlinton, Isaak Quast
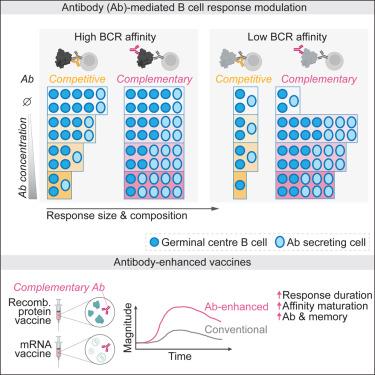
|
Existing antibodies (Abs) have varied effects on humoral immunity during subsequent infections. Here, we leveraged in vivo systems that allow precise control of antigen-specific Abs and B cells to examine the impact of Ab dose, affinity, and specificity in directing B cell activation and differentiation. Abs competing with the B cell receptor (BCR) epitope showed affinity-dependent suppression. By contrast, Abs targeting a complementary epitope, not overlapping with the BCR, shifted B cell differentiation toward Ab-secreting cells. Such Abs allowed for potent germinal center (GC) responses to otherwise poorly immunogenic sites by promoting antigen capture and presentation by low-affinity B cells. These mechanisms jointly diversified the B cell repertoire by facilitating the recruitment of high- and low-affinity B cells into Ab-secreting cell, GC, and memory B cell fates. Incorporation of small amounts of monoclonal Abs into protein- or mRNA-based vaccines enhanced immunogenicity and facilitated sustained immune responses, with implications for vaccine design and our understanding of protective immunity.
中文翻译:

通过具有表位互补性的抗体将疫苗从低免疫原性转化为高免疫原性
在随后的感染期间,现有的抗体 (Abs) 对体液免疫有不同的影响。在这里,我们利用 允许精确控制抗原特异性 Ab 和 B 细胞的体内系统来检查 Ab 剂量、亲和力和特异性对指导 B 细胞活化和分化的影响。与 B 细胞受体 (BCR) 表位竞争的 Abs 显示出亲和力依赖性抑制。相比之下,靶向互补表位的 Abs 与 BCR 不重叠,将 B 细胞分化转向分泌 Ab 的细胞。这种抗体通过促进低亲和力 B 细胞的抗原捕获和呈递,允许对免疫原性差的位点产生有效的生发中心 (GC) 反应。这些机制通过促进高亲和力和低亲和力 B 细胞募集到 Ab 分泌细胞、GC 和记忆 B 细胞命运中,共同使 B 细胞库多样化。在基于蛋白质或 mRNA 的疫苗中掺入少量单克隆抗体可增强免疫原性并促进持续的免疫反应,这对疫苗设计和我们对保护性免疫的理解具有重要意义。
更新日期:2024-09-20
中文翻译:

通过具有表位互补性的抗体将疫苗从低免疫原性转化为高免疫原性
在随后的感染期间,现有的抗体 (Abs) 对体液免疫有不同的影响。在这里,我们利用 允许精确控制抗原特异性 Ab 和 B 细胞的体内系统来检查 Ab 剂量、亲和力和特异性对指导 B 细胞活化和分化的影响。与 B 细胞受体 (BCR) 表位竞争的 Abs 显示出亲和力依赖性抑制。相比之下,靶向互补表位的 Abs 与 BCR 不重叠,将 B 细胞分化转向分泌 Ab 的细胞。这种抗体通过促进低亲和力 B 细胞的抗原捕获和呈递,允许对免疫原性差的位点产生有效的生发中心 (GC) 反应。这些机制通过促进高亲和力和低亲和力 B 细胞募集到 Ab 分泌细胞、GC 和记忆 B 细胞命运中,共同使 B 细胞库多样化。在基于蛋白质或 mRNA 的疫苗中掺入少量单克隆抗体可增强免疫原性并促进持续的免疫反应,这对疫苗设计和我们对保护性免疫的理解具有重要意义。






























 京公网安备 11010802027423号
京公网安备 11010802027423号