当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Breaking the final barrier: Evolution of cationic and ionizable lipid structure in lipid nanoparticles to escape the endosome
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-09-16 , DOI: 10.1016/j.addr.2024.115446 Kaitlin Mrksich 1 , Marshall S Padilla 1 , Michael J Mitchell 2
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-09-16 , DOI: 10.1016/j.addr.2024.115446 Kaitlin Mrksich 1 , Marshall S Padilla 1 , Michael J Mitchell 2
Affiliation
|
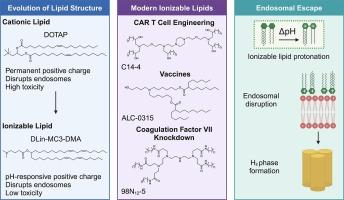
In the past decade, nucleic acid therapies have seen a boon in development and clinical translation largely due to advances in nanotechnology that have enabled their safe and targeted delivery. Nanoparticles can protect nucleic acids from degradation by serum enzymes and can facilitate entry into cells. Still, achieving endosomal escape to allow nucleic acids to enter the cytoplasm has remained a significant barrier, where less than 5% of nanoparticles within the endo -lysosomal pathway are able to transfer their cargo to the cytosol. Lipid-based drug delivery vehicles, particularly lipid nanoparticles (LNPs), have been optimized to achieve potent endosomal escape, and thus have been the vector of choice in the clinic as demonstrated by their utilization in the COVID-19 mRNA vaccines. The success of LNPs is in large part due to the rational design of lipids that can specifically overcome endosomal barriers. In this review, we chart the evolution of lipid structure from cationic lipids to ionizable lipids, focusing on structure–function relationships, with a focus on how they relate to endosomal escape. Additionally, we examine recent advancements in ionizable lipid structure as well as discuss the future of lipid design.
中文翻译:

打破最终屏障:脂质纳米颗粒中阳离子和可电离脂质结构的进化以逃避内体
在过去十年中,核酸疗法在开发和临床转化方面取得了长足的进步,这主要是由于纳米技术的进步使其能够安全和靶向地递送。纳米颗粒可以保护核酸不被血清酶降解,并可以促进进入细胞。尽管如此,实现内体逃逸以允许核酸进入细胞质仍然是一个重要的障碍,其中内溶酶体途径中只有不到 5% 的纳米颗粒能够将其货物转移到胞质溶胶中。基于脂质的药物递送载体,特别是脂质纳米颗粒 (LNP),已经过优化以实现有效的内体逃逸,因此已成为临床上的首选载体,它们在 COVID-19 mRNA 疫苗中的使用证明了这一点。LNP 的成功在很大程度上归功于可以特异性克服内体障碍的脂质的合理设计。在这篇综述中,我们绘制了脂质结构从阳离子脂质到可电离脂质的演变,重点关注结构-功能关系,重点是它们与内体逃逸的关系。此外,我们还研究了可电离脂质结构的最新进展,并讨论了脂质设计的未来。
更新日期:2024-09-16
中文翻译:

打破最终屏障:脂质纳米颗粒中阳离子和可电离脂质结构的进化以逃避内体
在过去十年中,核酸疗法在开发和临床转化方面取得了长足的进步,这主要是由于纳米技术的进步使其能够安全和靶向地递送。纳米颗粒可以保护核酸不被血清酶降解,并可以促进进入细胞。尽管如此,实现内体逃逸以允许核酸进入细胞质仍然是一个重要的障碍,其中内溶酶体途径中只有不到 5% 的纳米颗粒能够将其货物转移到胞质溶胶中。基于脂质的药物递送载体,特别是脂质纳米颗粒 (LNP),已经过优化以实现有效的内体逃逸,因此已成为临床上的首选载体,它们在 COVID-19 mRNA 疫苗中的使用证明了这一点。LNP 的成功在很大程度上归功于可以特异性克服内体障碍的脂质的合理设计。在这篇综述中,我们绘制了脂质结构从阳离子脂质到可电离脂质的演变,重点关注结构-功能关系,重点是它们与内体逃逸的关系。此外,我们还研究了可电离脂质结构的最新进展,并讨论了脂质设计的未来。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号