当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small Ring Molecules Comprising 3–6 Boron Atoms: An Account on Synthesis, Structure, and Orbital Engineering
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/acs.accounts.4c00497
Sourav Kar 1 , Subhash Bairagi 1 , Gaurav Joshi 2 , Eluvathingal D Jemmis 2 , Hans-Jörg Himmel 3 , Sundargopal Ghosh 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/acs.accounts.4c00497
Sourav Kar 1 , Subhash Bairagi 1 , Gaurav Joshi 2 , Eluvathingal D Jemmis 2 , Hans-Jörg Himmel 3 , Sundargopal Ghosh 1
Affiliation
|
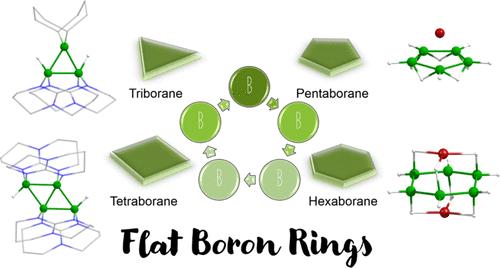
Unlike carbon, boron does not usually form ring compounds due to its electron-deficiency-driven affinity toward polyhedral geometries. The polyhedral boranes having closo-, nido-, arachno-, or hypho-shapes can be structurally and electronically correlated using various electron counting rules developed by Wade, Mingos, and one of us. However, in the last few decades, boron chemistry progressed significantly toward ring systems. In this regard, three of our research groups have made significant contributions to the development of boron ring molecules through different synthetic approaches. While the Ghosh group generally starts from transition metal (TM) stabilized boron species, the Himmel group typically starts from electron-deficient TM-free boron ring compounds. On the other hand, the Jemmis group studies boron rings and their analogous structures computationally and develops electron counting rules to describe them. Over the past few years, through different synthetic approaches, several boron ring molecules have been prepared by our research groups and others. Recently, the Ghosh group has reported the synthesis of an almost planar B6-ring that is stabilized by a TM template. Similarly, the B3-, B4-, and B5-rings have also been stabilized in the coordination spheres of early and late TMs. The recent work of Himmel has uncovered some remarkable diversity in the structures and bonding of B3 and B4 rings, along with their redox reactions. The well-known hydrocarbon analogues of these borane rings, i.e., two-dimensional aromatic compounds [C3H3]+, [C5H5]−, [C6H6], etc., are governed by Hückel’s (4n + 2) π-electron rule. However, planar or nearly planar borane rings are not seriously thought of as achievable targets. One of the reasons for this is the influence of the Rudolph diagram in the thought process of chemists that the nido- and arachno-structures generated from closo-polyhedral boranes must also be three-dimensional (3D) fragments. However, this is not the only possibility. Flat arachno- and nido-boranes reminiscent of their organic counterparts follow from an equivalent of the Rudolph diagram. Therefore, this Account is very much necessary for the boron community, in particular, to design and synthesize 3–6 membered boron rings or beyond. This Account aims to highlight significant ongoing experimental and theoretical results in this area from our groups, in addition to relevant works from other groups wherever appropriate. This will also bring into focus various ways in which the flat Bn-systems can be stabilized, such as the utilization of TM or main group caps, utilization of various Lewis bases, edge-condensation of small rings, control over the electron count, and orbital engineering.
中文翻译:

包含 3-6 个硼原子的小环分子:合成、结构和轨道工程说明
与碳不同,硼通常不会形成环化合物,因为它对多面体几何形状的电子缺陷驱动的亲和力。具有 closo、nido、arachno 或 hypho 形状的多面体硼烷可以使用 Wade、Mingos 和我们中的一个人开发的各种电子计数规则在结构和电子上相关。然而,在过去的几十年里,硼化学显着地朝着环系发展。在这方面,我们的三个研究小组通过不同的合成方法为硼环分子的开发做出了重大贡献。Ghosh 组通常从过渡金属 (TM) 稳定的硼种类开始,而 Himmel 组通常从缺电子的 TM 无硼环化合物开始。另一方面,Jemmis 小组通过计算研究硼环及其类似结构,并开发电子计数规则来描述它们。在过去的几年里,通过不同的合成方法,我们的研究小组和其他人已经制备了几种硼环分子。最近,Ghosh 小组报道了由 TM 模板稳定的几乎平面的 B6 环的合成。同样,B3-、B4-和 B5-环也稳定在早期和晚期 TM 的配位球中。Himmel 最近的工作揭示了 B3 和 B4 环的结构和键合以及它们的氧化还原反应的一些显着多样性。这些硼烷环的众所周知的碳氢化合物类似物,即、二维芳香族化合物 [C3H3]+、[C5H5]−、[C6H6] 等受 Hückel (4n + 2) π电子规则控制。然而,平面或接近平面的硼烷环并没有被认真地视为可实现的目标。造成这种情况的原因之一是鲁道夫图在化学家的思维过程中的影响,即由梭形多面体硼烷产生的 nido 和 arachno 结构也必须是三维 (3D) 片段。然而,这并不是唯一的可能性。扁平的蛛形纲和钰基硼烷让人想起它们的有机对应物,相当于鲁道夫图。因此,这个账户对于硼界来说非常必要,特别是设计和合成 3-6 元硼环或更多。本账户旨在突出我们小组在该领域正在进行的重要实验和理论成果,以及其他小组的相关工作(如适用)。这也将使稳定平坦 Bn 系统的各种方式成为焦点,例如使用 TM 或主族帽、利用各种路易斯碱、小环的边缘凝聚、电子数控制和轨道工程。
更新日期:2024-09-18
中文翻译:

包含 3-6 个硼原子的小环分子:合成、结构和轨道工程说明
与碳不同,硼通常不会形成环化合物,因为它对多面体几何形状的电子缺陷驱动的亲和力。具有 closo、nido、arachno 或 hypho 形状的多面体硼烷可以使用 Wade、Mingos 和我们中的一个人开发的各种电子计数规则在结构和电子上相关。然而,在过去的几十年里,硼化学显着地朝着环系发展。在这方面,我们的三个研究小组通过不同的合成方法为硼环分子的开发做出了重大贡献。Ghosh 组通常从过渡金属 (TM) 稳定的硼种类开始,而 Himmel 组通常从缺电子的 TM 无硼环化合物开始。另一方面,Jemmis 小组通过计算研究硼环及其类似结构,并开发电子计数规则来描述它们。在过去的几年里,通过不同的合成方法,我们的研究小组和其他人已经制备了几种硼环分子。最近,Ghosh 小组报道了由 TM 模板稳定的几乎平面的 B6 环的合成。同样,B3-、B4-和 B5-环也稳定在早期和晚期 TM 的配位球中。Himmel 最近的工作揭示了 B3 和 B4 环的结构和键合以及它们的氧化还原反应的一些显着多样性。这些硼烷环的众所周知的碳氢化合物类似物,即、二维芳香族化合物 [C3H3]+、[C5H5]−、[C6H6] 等受 Hückel (4n + 2) π电子规则控制。然而,平面或接近平面的硼烷环并没有被认真地视为可实现的目标。造成这种情况的原因之一是鲁道夫图在化学家的思维过程中的影响,即由梭形多面体硼烷产生的 nido 和 arachno 结构也必须是三维 (3D) 片段。然而,这并不是唯一的可能性。扁平的蛛形纲和钰基硼烷让人想起它们的有机对应物,相当于鲁道夫图。因此,这个账户对于硼界来说非常必要,特别是设计和合成 3-6 元硼环或更多。本账户旨在突出我们小组在该领域正在进行的重要实验和理论成果,以及其他小组的相关工作(如适用)。这也将使稳定平坦 Bn 系统的各种方式成为焦点,例如使用 TM 或主族帽、利用各种路易斯碱、小环的边缘凝聚、电子数控制和轨道工程。




































 京公网安备 11010802027423号
京公网安备 11010802027423号