Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2024-09-19 , DOI: 10.3762/bjoc.20.202
Daria A. Burmistrova, Andrey Galustyan, Nadezhda P. Pomortseva, Kristina D. Pashaeva, Maxim V. Arsenyev, Oleg P. Demidov, Mikhail A. Kiskin, Andrey I. Poddel’sky, Nadezhda T. Berberova, Ivan V. Smolyaninov
Abstract
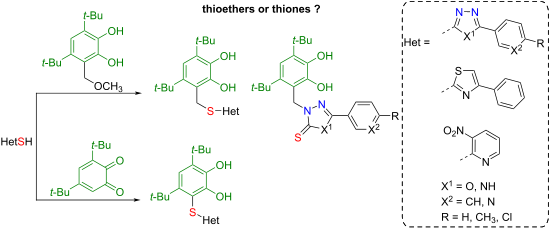
A series of new RS−, RS−CH2− and R2N−CH2-functionalized сatechols with heterocyclic fragments such as 1,3,4-oxadiazole, 1,2,4-triazole, thiazole, or pyridine were synthesized by the reaction of 3,5-di-tert-butyl-o-benzoquinone or 3,5-di-tert-butyl-6-methoxymethylcatechol with different heterocyclic thiols. The S-functionalized catechols were prepared by the Michael reaction from 3,5-di-tert-butyl-o-benzoquinone and the corresponding thiols. The starting reagents such as substituted 1,3,4-oxadiazole-2-thiols and 4H-triazole-3-thiols are characterized by thiol–thione tautomerism, therefore their reactions with 3,5-di-tert-butyl-6-methoxymethylcatechol can proceed at the sulfur or nitrogen atom. In the case of mercapto-derivatives of thiazole or pyridine, this process leads to the formation of the corresponding thioethers with a methylene linker. At the same time, thiolated 1,3,4-oxadiazole or 1,2,4-triazole undergo alkylation at the nitrogen atom in the reaction with 3,5-di-tert-butyl-6-methoxymethylcatechol to form the corresponding thiones. The yield of reaction products ranges from 42 to 80%. The crystal structures of catechols with 3-nitropyridine or 1,3,4-oxadiazole-2(3H)-thione moieties were established by single-crystal X-ray analysis. The possibility of forming intra- and intermolecular hydrogen bonds has been established for these compounds. The electrochemical behavior of the studied compounds is influenced by several factors: the nature of the heterocycle and its substituents, the presence of a sulfur atom in the catechol ring, or a thione group in the heterocyclic core. The radical scavenging activity and antioxidant properties were determined using the reaction with synthetic radicals, the cupric reducing antioxidant capacity assay, the inhibition process of superoxide radical anion formation by xanthine oxidase, and the process of lipid peroxidation of rat liver (Wistar) homogenates in vitro.
Beilstein J. Org. Chem. 2024, 20, 2378–2391. doi:10.3762/bjoc.20.202
中文翻译:

1,3,4-恶二唑、1,2,4-三唑、噻唑或吡啶片段位阻儿茶酚的合成、电化学性质和抗氧化活性
抽象的
一系列具有杂环片段(如1,3,4-恶二唑、1,2,4-三唑、噻唑或吡啶)的新型RS−、RS−CH 2 − 和R 2 N−CH 2官能化儿茶酚通过3,5-二叔丁基-邻苯醌或3,5-二叔丁基-6-甲氧基甲基儿茶酚与不同杂环硫醇的反应。 S-官能化儿茶酚是通过迈克尔反应从3,5-二叔丁基-邻苯醌和相应的硫醇制备的。起始试剂如取代的1,3,4-恶二唑-2-硫醇和4H-三唑-3-硫醇具有硫醇-硫酮互变异构的特征,因此它们与3,5-二叔丁基-6-反应甲氧基甲基儿茶酚可以在硫或氮原子上进行。在噻唑或吡啶的巯基衍生物的情况下,该过程导致与亚甲基连接体形成相应的硫醚。同时,硫醇化的1,3,4-恶二唑或1,2,4-三唑在与3,5-二叔丁基-6-甲氧基甲基儿茶酚的反应中在氮原子上发生烷基化,形成相应的硫酮。反应产物的收率在42%至80%之间。通过单晶X射线分析建立了具有3-硝基吡啶或1,3,4-恶二唑-2( 3H )-硫酮部分的儿茶酚的晶体结构。已经确定这些化合物形成分子内和分子间氢键的可能性。所研究化合物的电化学行为受多种因素影响:杂环及其取代基的性质、儿茶酚环中硫原子的存在或杂环核中硫酮基团的存在。 通过与合成自由基的反应、铜还原抗氧化能力测定、黄嘌呤氧化酶抑制超氧自由基阴离子形成的过程以及体外大鼠肝( Wistar )匀浆的脂质过氧化过程来测定自由基清除活性和抗氧化特性。


































 京公网安备 11010802027423号
京公网安备 11010802027423号