当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elastase-targeting biomimic nanoplatform for neurovascular remodeling by inhibiting NETosis mediated AlM2 inflammasome activation in ischemic stroke
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-09-19 , DOI: 10.1016/j.jconrel.2024.09.026 Chunming Tang 1 , Feng Jia 2 , Min Wu 1 , Yanling Wang 1 , Xiaowei Lu 3 , Jinyu Li 1 , Yan Ding 1 , Weilin Chen 1 , Xufeng Chen 4 , Feng Han 1 , Huae Xu 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-09-19 , DOI: 10.1016/j.jconrel.2024.09.026 Chunming Tang 1 , Feng Jia 2 , Min Wu 1 , Yanling Wang 1 , Xiaowei Lu 3 , Jinyu Li 1 , Yan Ding 1 , Weilin Chen 1 , Xufeng Chen 4 , Feng Han 1 , Huae Xu 1
Affiliation
|
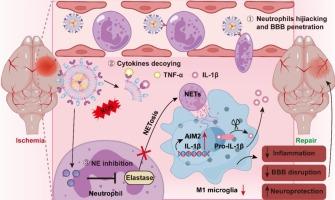
Neutrophil elastase (NE) is a protease released by activated neutrophils in the brain parenchyma after cerebral ischemia, which plays a pivotal role in the regulation of neutrophil extracellular traps (NETs) formation. The excess NETs could lead to blood-brain barrier (BBB) breakdown, overwhelming neuroinflammation, and neuronal injury. While the potential of targeting neutrophils and inhibiting NE activity to mitigate ischemic stroke (IS) pathology has been recognized, effective strategies that inhibit NETs formation remain under-explored. Herein, a biomimic multifunctional nanoplatform (HM@ST/TeTeLipos) was developed for active NE targeting and IS treatment. The core of the HM@ST/TeTeLipos consisted of sivelestat-loaded ditelluride-containing liposomes with ROS-responsive and NE-inhibiting properties. The outer shell was composed of platelet-neutrophil hybrid membrane vesicles (HMVs), which acted to hijack neutrophils and neutralize proinflammatory cytokines. Our studies revealed that HM@ST/TeTeLipos could effectively inhibit NE activity, thereby suppressing the release of NETs, impeding the activation of the AIM2 inflammasome, and consequently redirecting the immune response away from a pro-inflammatory M1 microglia phenotype. This resulted in enhanced neurovascular remodeling, reduced BBB disruption, and diminished neuroinflammation, ultimately promoting neuron survival. We believe that this innovative approach holds significant potential for improving the treatment of IS and various NE-mediated inflammatory diseases.
中文翻译:

通过抑制缺血性脑卒中中 NETosis 介导的 AlM2 炎性小体激活,靶向弹性蛋白酶的仿生纳米平台用于神经血管重塑
中性粒细胞弹性蛋白酶 (NE) 是脑缺血后脑实质中活化的中性粒细胞释放的一种蛋白酶,在调节中性粒细胞胞外陷阱 (NETs) 的形成中起关键作用。过量的 NET 可能导致血脑屏障 (BBB) 崩溃、压倒性的神经炎症和神经元损伤。虽然靶向中性粒细胞和抑制 NE 活性以减轻缺血性中风 (IS) 病理的潜力已得到认可,但抑制 NETs 形成的有效策略仍未得到充分探索。在此,开发了一种用于主动 NE 靶向和 IS 治疗的仿生多功能纳米平台 (HM@ST/TeTeLipos)。HM@ST/TeTeLipos 的核心由载有 sivelestat 的含二碲化物的脂质体组成,具有 ROS 反应和 NE 抑制特性。外壳由血小板-中性粒细胞杂交膜囊泡 (HMV) 组成,其作用是劫持中性粒细胞并中和促炎细胞因子。我们的研究表明,HM@ST/TeTeLipos 可以有效抑制 NE 活性,从而抑制 NETs 的释放,阻碍 AIM2 炎性小体的激活,从而将免疫反应从促炎性 M1 小胶质细胞表型转移开。这导致神经血管重塑增强,减少 BBB 破坏,减少神经炎症,最终促进神经元存活。我们相信,这种创新方法在改善 IS 和各种 NE 介导的炎症性疾病的治疗方面具有巨大潜力。
更新日期:2024-09-19
中文翻译:

通过抑制缺血性脑卒中中 NETosis 介导的 AlM2 炎性小体激活,靶向弹性蛋白酶的仿生纳米平台用于神经血管重塑
中性粒细胞弹性蛋白酶 (NE) 是脑缺血后脑实质中活化的中性粒细胞释放的一种蛋白酶,在调节中性粒细胞胞外陷阱 (NETs) 的形成中起关键作用。过量的 NET 可能导致血脑屏障 (BBB) 崩溃、压倒性的神经炎症和神经元损伤。虽然靶向中性粒细胞和抑制 NE 活性以减轻缺血性中风 (IS) 病理的潜力已得到认可,但抑制 NETs 形成的有效策略仍未得到充分探索。在此,开发了一种用于主动 NE 靶向和 IS 治疗的仿生多功能纳米平台 (HM@ST/TeTeLipos)。HM@ST/TeTeLipos 的核心由载有 sivelestat 的含二碲化物的脂质体组成,具有 ROS 反应和 NE 抑制特性。外壳由血小板-中性粒细胞杂交膜囊泡 (HMV) 组成,其作用是劫持中性粒细胞并中和促炎细胞因子。我们的研究表明,HM@ST/TeTeLipos 可以有效抑制 NE 活性,从而抑制 NETs 的释放,阻碍 AIM2 炎性小体的激活,从而将免疫反应从促炎性 M1 小胶质细胞表型转移开。这导致神经血管重塑增强,减少 BBB 破坏,减少神经炎症,最终促进神经元存活。我们相信,这种创新方法在改善 IS 和各种 NE 介导的炎症性疾病的治疗方面具有巨大潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号