当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-substituted-4-(pyridin-4-ylalkyl)piperazine-1-carboxamides and related compounds as Leishmania CYP51 and CYP5122A1 inhibitors
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2024-09-06 , DOI: 10.1016/j.bmc.2024.117907 Chris La Rosa 1 , Pankaj Sharma 1 , M Junaid Dar 1 , Yiru Jin 2 , Lingli Qin 2 , Anuradha Roy 3 , Allie Kendall 1 , Meng Wu 4 , Zhihong Lin 5 , Dmitriy Uchenik 6 , Junan Li 6 , Somrita Basu 7 , Samrat Moitra 7 , Kai Zhang 7 , Michael Zhuo Wang 2 , Karl A Werbovetz 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2024-09-06 , DOI: 10.1016/j.bmc.2024.117907 Chris La Rosa 1 , Pankaj Sharma 1 , M Junaid Dar 1 , Yiru Jin 2 , Lingli Qin 2 , Anuradha Roy 3 , Allie Kendall 1 , Meng Wu 4 , Zhihong Lin 5 , Dmitriy Uchenik 6 , Junan Li 6 , Somrita Basu 7 , Samrat Moitra 7 , Kai Zhang 7 , Michael Zhuo Wang 2 , Karl A Werbovetz 1
Affiliation
|
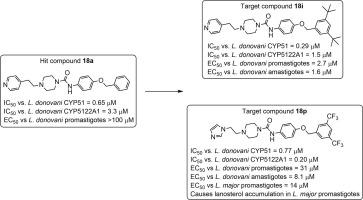
CYP5122A1, an enzyme involved in sterol biosynthesis in Leishmania , was recently characterized as a sterol C4-methyl oxidase. Screening of a library of compounds against CYP5122A1 and CYP51 from Leishmania resulted in the identification of two structurally related classes of inhibitors of these enzymes. Analogs of screening hit N -(3,5-dimethylphenyl)-4-(pyridin-4-ylmethyl)piperazine-1-carboxamide (4a ) were generally strong inhibitors of CYP51 but were less potent against CYP5122A1 and typically displayed weak inhibition of L. donovani promastigote growth. Analogs of screening hit N -(4-(benzyloxy)phenyl)-4-(2-(pyridin-4-yl)ethyl)piperazine-1-carboxamide (18a ) were stronger inhibitors of both CYP5122A1 and L. donovani promastigote proliferation but also remained selective for inhibition of CYP51. Two compounds in this series, N -(4-((3,5-bis(trifluoromethyl)benzyl)oxy)phenyl)-4-(2-(pyridin-4-yl)ethyl)piperazine-1-carboxamide (18e ) and N -(4-((3,5-di-tert-butylbenzyl)oxy)phenyl)-4-(2-(pyridin-4-yl)ethyl)piperazine-1-carboxamide (18i ) showed modest selectivity for inhibiting L. donovani promastigote proliferation compared to J774 macrophages and were effective against intracellular L. donovani with EC50 values in the low micromolar range. Replacement of the 4-pyridyl ring present in 18e with imidazole resulted in a compound (4-(2-(1H -imidazol-1-yl)ethyl)-N -(4-((3,5-bis(trifluoromethyl)benzyl)oxy)phenyl)piperazine-1-carboxamide, 18p ) with approximately fourfold selectivity for CYP5122A1 over CYP51 that inhibited both enzymes with IC50 values ≤ 1 µM, although selective potency against L. donovani promastigotes was lost. Compound 18p also inhibited the proliferation of L. major promastigotes and caused the accumulation of 4-methylated sterols in L. major membranes, indicating that this compound blocks sterol demethylation at the 4-position in Leishmania parasites. The molecules described here may therefore be useful for the future identification of dual inhibitors of CYP51 and CYP5122A1 as potential antileishmanial drug candidates and as probes to shed further light on sterol biosynthesis in Leishmania and related parasites.
中文翻译:

N-取代-4-(吡啶-4-烷基)哌嗪-1-甲酰胺和相关化合物,如利什曼原虫 CYP51 和 CYP5122A1 抑制剂
CYP5122A1 是一种参与利什曼原虫甾醇生物合成的酶,最近被表征为甾醇 C4-甲基氧化酶。筛选来自利什曼原虫的 CYP5122A1 和 CYP51 的化合物库,鉴定出这些酶的两类结构相关的抑制剂。筛选命中 N-(3,5-二甲基苯基)-4-(吡啶-4-基甲基)哌嗪-1-甲酰胺 (4a) 的类似物通常是 CYP51 的强抑制剂,但对 CYP5122A1 的效力较弱,并且通常表现出对 L. donovani 前鞭毛体生长的弱抑制。筛选命中 N-(4-(苄氧基)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18a) 的类似物是 CYP5122A1 和 L. donovani 前鞭毛体增殖的更强抑制剂,但对抑制 CYP51 仍然具有选择性。该系列中的两种化合物,N-(4-((3,5-双(三氟甲基)苄基)氧基)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18e) 和 N-(4-((3,5-二叔丁基苄基)氧)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18i) 与 J774 巨噬细胞相比,对抑制多诺瓦尼乳杆菌前鞭毛体增殖表现出适度的选择性,并且对细胞内多诺瓦尼乳腺乳杆菌有效,EC50 值在低微摩尔范围内。用咪唑取代 18e 中存在的 4-吡啶环产生化合物(4-(2-(1H-咪唑-1-基)乙基)-N-(4-((3,5-双(三氟甲基)苄基)氧)苯基)哌嗪-1-甲酰胺),对 CYP5122A1 的选择性约为 CYP51 的四倍,抑制两种酶的 IC50 值≤ 1 μM,尽管对 L. donovani 前鞭毛体的选择性丧失。化合物 18p 还抑制 L. major 前鞭毛体的增殖,并导致 4-甲基化甾醇在 L 中积累。 主膜,表明该化合物阻断利什曼原虫寄生虫中 4 位的甾醇去甲基化。因此,此处描述的分子可能有助于未来鉴定 CYP51 和 CYP5122A1 的双重抑制剂作为潜在的抗利什曼原虫候选药物,并作为探针进一步阐明利什曼原虫和相关寄生虫中甾醇的生物合成。
更新日期:2024-09-06
中文翻译:

N-取代-4-(吡啶-4-烷基)哌嗪-1-甲酰胺和相关化合物,如利什曼原虫 CYP51 和 CYP5122A1 抑制剂
CYP5122A1 是一种参与利什曼原虫甾醇生物合成的酶,最近被表征为甾醇 C4-甲基氧化酶。筛选来自利什曼原虫的 CYP5122A1 和 CYP51 的化合物库,鉴定出这些酶的两类结构相关的抑制剂。筛选命中 N-(3,5-二甲基苯基)-4-(吡啶-4-基甲基)哌嗪-1-甲酰胺 (4a) 的类似物通常是 CYP51 的强抑制剂,但对 CYP5122A1 的效力较弱,并且通常表现出对 L. donovani 前鞭毛体生长的弱抑制。筛选命中 N-(4-(苄氧基)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18a) 的类似物是 CYP5122A1 和 L. donovani 前鞭毛体增殖的更强抑制剂,但对抑制 CYP51 仍然具有选择性。该系列中的两种化合物,N-(4-((3,5-双(三氟甲基)苄基)氧基)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18e) 和 N-(4-((3,5-二叔丁基苄基)氧)苯基)-4-(2-(吡啶-4-基)乙基)哌嗪-1-甲酰胺 (18i) 与 J774 巨噬细胞相比,对抑制多诺瓦尼乳杆菌前鞭毛体增殖表现出适度的选择性,并且对细胞内多诺瓦尼乳腺乳杆菌有效,EC50 值在低微摩尔范围内。用咪唑取代 18e 中存在的 4-吡啶环产生化合物(4-(2-(1H-咪唑-1-基)乙基)-N-(4-((3,5-双(三氟甲基)苄基)氧)苯基)哌嗪-1-甲酰胺),对 CYP5122A1 的选择性约为 CYP51 的四倍,抑制两种酶的 IC50 值≤ 1 μM,尽管对 L. donovani 前鞭毛体的选择性丧失。化合物 18p 还抑制 L. major 前鞭毛体的增殖,并导致 4-甲基化甾醇在 L 中积累。 主膜,表明该化合物阻断利什曼原虫寄生虫中 4 位的甾醇去甲基化。因此,此处描述的分子可能有助于未来鉴定 CYP51 和 CYP5122A1 的双重抑制剂作为潜在的抗利什曼原虫候选药物,并作为探针进一步阐明利什曼原虫和相关寄生虫中甾醇的生物合成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号