当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Entries in Organocatalysts from an Alkaloid Library; Development of Aminal Catalysis for a Michael Reaction Based on Calycanthine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/jacs.4c10242
Kyosuke Yamanishi 1 , Gin Ashihara 1 , Shinya Shiomi 1 , Shingo Harada 1 , Mariko Kitajima 1 , Hiromitsu Takayama 1 , Hayato Ishikawa 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/jacs.4c10242
Kyosuke Yamanishi 1 , Gin Ashihara 1 , Shinya Shiomi 1 , Shingo Harada 1 , Mariko Kitajima 1 , Hiromitsu Takayama 1 , Hayato Ishikawa 1
Affiliation
|
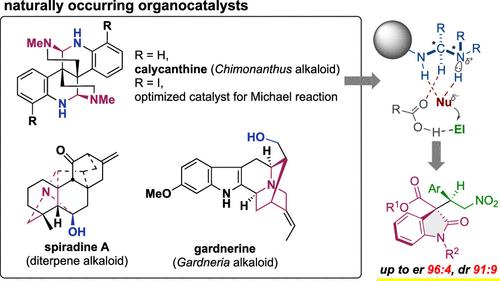
Natural products have historically been actively evaluated for their biological activity in the development of pharmaceuticals, while their evaluation as asymmetric catalysts has rarely been explored. In this study, we evaluated the catalytic activity of the natural product library. Three naturally occurring alkaloids, gardnerine, spiradine A, and calycanthine, were found to catalyze an asymmetric Michael reaction using oxindole and nitrostyrene. We further studied (+)-calycanthine, which is characterized by its aminal structure. Concise synthetic and extraction protocols were developed to provide both enantiomers of calycanthine. Further derivatization of this alkaloid led to improved enantioselectivity in a model reaction. Computational studies suggested that the aminal moiety of the catalyst activated nucleophiles and electrophiles through multiple hydrogen bonding interactions, including nonclassical hydrogen bonds between carboxylic acid and the aminal C–H.
中文翻译:

生物碱库中有机催化剂的新条目;基于花蜡质的迈克尔反应缩醛胺催化的研究进展
历史上,天然产物在药物开发中的生物活性一直被积极评估,但很少探索它们作为不对称催化剂的评估。在这项研究中,我们评估了天然产物库的催化活性。三种天然存在的生物碱,加德纳碱、螺吡啶 A 和花萼花碱,被发现可催化使用羟吲哚和硝基苯乙烯的不对称迈克尔反应。我们进一步研究了(+)-花蕾角碱,其特征在于其缩醛胺结构。开发了简明的合成和提取方案以提供花盏素的两种对映体。这种生物碱的进一步衍生化提高了模型反应中的对映选择性。计算研究表明,催化剂的缩醛胺部分通过多种氢键相互作用(包括羧酸和缩醛胺 C-H 之间的非经典氢键)激活亲核试剂和亲电子试剂。
更新日期:2024-09-18
中文翻译:

生物碱库中有机催化剂的新条目;基于花蜡质的迈克尔反应缩醛胺催化的研究进展
历史上,天然产物在药物开发中的生物活性一直被积极评估,但很少探索它们作为不对称催化剂的评估。在这项研究中,我们评估了天然产物库的催化活性。三种天然存在的生物碱,加德纳碱、螺吡啶 A 和花萼花碱,被发现可催化使用羟吲哚和硝基苯乙烯的不对称迈克尔反应。我们进一步研究了(+)-花蕾角碱,其特征在于其缩醛胺结构。开发了简明的合成和提取方案以提供花盏素的两种对映体。这种生物碱的进一步衍生化提高了模型反应中的对映选择性。计算研究表明,催化剂的缩醛胺部分通过多种氢键相互作用(包括羧酸和缩醛胺 C-H 之间的非经典氢键)激活亲核试剂和亲电子试剂。


































 京公网安备 11010802027423号
京公网安备 11010802027423号