当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Chiral Endocyclic Allenes and Alkynes via Pd-Catalyzed Asymmetric Higher-Order Dipolar Cycloaddition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/jacs.4c10328 Bin Shi 1 , Meng Xiao 1 , Jin-Pu Zhao 1 , Zhihan Zhang 1 , Wen-Jing Xiao 1, 2 , Liang-Qiu Lu 1, 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-18 , DOI: 10.1021/jacs.4c10328 Bin Shi 1 , Meng Xiao 1 , Jin-Pu Zhao 1 , Zhihan Zhang 1 , Wen-Jing Xiao 1, 2 , Liang-Qiu Lu 1, 3, 4
Affiliation
|
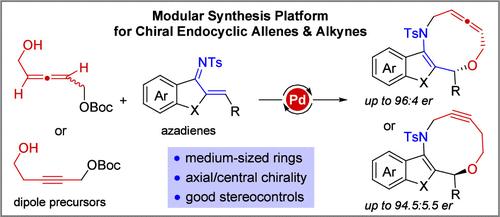
A Pd-catalyzed asymmetric higher-order dipolar cycloaddition between allenyl carbonates and azadienes is achieved by exploiting novel alkylidene-π-allyl-Pd dipoles. This research provides a modular platform for the synthesis of challenging chiral endocyclic allenes bearing a medium-sized heterocyclic motif and a centrally chiral stereocenter in good yields with high enantio- and diastereoselectivities (29 examples, up to 97% yield, 97:3 er and >19:1 dr). Experimental and computational studies elucidate the possible reaction mechanism and the observed stereochemical results. Based on the mechanistic understanding, a new π-propargyl-Pd dipole was designed to further extend the success of the higher order dipolar cycloaddition strategy to the synthesis of 10-membered endocyclic alkynes from propargyl carbonates and azadienes (13 examples, up to 98% yield and 94.5:5.5 er).
中文翻译:

Pd 催化不对称高阶偶极环加成合成手性环内烯和炔
通过利用新型亚烷基-π-烯丙基-Pd偶极子实现了碳酸丙二烯酯和氮杂二烯之间的Pd催化的不对称高阶偶极环加成反应。这项研究提供了一个模块化平台,用于合成具有中等大小杂环基序和中心手性立构中心的具有挑战性的手性环内丙二烯,其产率良好,具有高对映体和非对映选择性(29个实例,产率高达97%,97:3 er和>19:1 博士)。实验和计算研究阐明了可能的反应机制和观察到的立体化学结果。基于机理的理解,设计了一种新的π-炔丙基-Pd偶极子,以进一步将高阶偶极环加成策略的成功扩展到由碳酸炔丙酯和氮杂二烯合成10元环内炔烃(13个例子,高达98%)产率和 94.5:5.5 er)。
更新日期:2024-09-18
中文翻译:

Pd 催化不对称高阶偶极环加成合成手性环内烯和炔
通过利用新型亚烷基-π-烯丙基-Pd偶极子实现了碳酸丙二烯酯和氮杂二烯之间的Pd催化的不对称高阶偶极环加成反应。这项研究提供了一个模块化平台,用于合成具有中等大小杂环基序和中心手性立构中心的具有挑战性的手性环内丙二烯,其产率良好,具有高对映体和非对映选择性(29个实例,产率高达97%,97:3 er和>19:1 博士)。实验和计算研究阐明了可能的反应机制和观察到的立体化学结果。基于机理的理解,设计了一种新的π-炔丙基-Pd偶极子,以进一步将高阶偶极环加成策略的成功扩展到由碳酸炔丙酯和氮杂二烯合成10元环内炔烃(13个例子,高达98%)产率和 94.5:5.5 er)。































 京公网安备 11010802027423号
京公网安备 11010802027423号