当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, Antifungal Evaluation, and Three-Dimensional Quantitative Structure–Activity Relationship of Novel 5-Sulfonyl-1,3,4-thiadiazole Flavonoids
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-17 , DOI: 10.1021/acs.jafc.4c03505 Peng Dai 1 , Yufei Li 1 , Zihua Ma 1 , Jian Jiao 1 , Qing Xia 1 , Weihua Zhang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-17 , DOI: 10.1021/acs.jafc.4c03505 Peng Dai 1 , Yufei Li 1 , Zihua Ma 1 , Jian Jiao 1 , Qing Xia 1 , Weihua Zhang 1
Affiliation
|
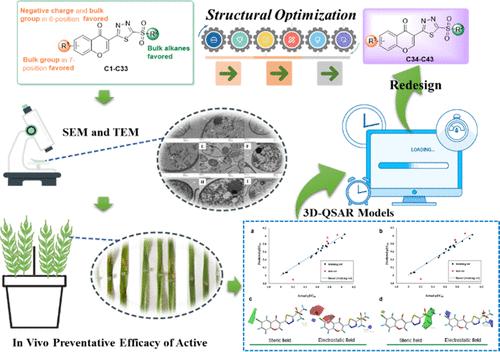
Plant pathogenic fungi frequently disrupt the normal physiological and biochemical functions of plants, leading to diseases, compromising plant health, and ultimately reducing crop yield. This study aimed to address this challenge by identifying antifungal agents with innovative structures and novel mechanisms of action. We designed and synthesized a series of flavonoid derivatives substituted with 5-sulfonyl-1,3,4-thiadiazole and evaluated their antifungal activity against five phytopathogenic fungi. Most flavonoid derivatives demonstrated excellent antifungal activity against Botrytis cinerea (B. cinerea), Alternaria solani (A. solani), Rhizoctorzia solani (R. solani), Fusarium graminearum (F. graminearum), and Colletotrichum orbiculare (C. orbiculare). Specifically, the EC50 values of 38 target compounds against R. solani were below 4 μg/mL, among which the compounds C13 (EC50 = 0.49 μg/mL), C15 (EC50 = 0.37 μg/mL), and C19 (EC50 = 0.37 μg/mL) had the most prominent antifungal activity, superior to that of the control drug carbendazim (EC50 = 0.52 μg/mL). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the cellular ultrastructures of R. solani mycelia and cells after treatment with the compound C19 revealed sprawling growth of hyphae, a distorted outline of their cell walls, and reduced mitochondrial numbers. Studying the 3D-QSAR between the molecular structure and antifungal activity of 5-sulfonyl-1,3,4-thiadiazole-substituted flavonoid derivatives could significantly improve conventional drug molecular design pathways and facilitate the development of novel antifungal leads.
中文翻译:

新型 5-磺酰基-1,3,4-噻二唑类黄酮的设计、合成、抗真菌评价和三维定量构效关系
植物病原真菌经常破坏植物的正常生理生化功能,导致疾病,损害植物健康,最终降低作物产量。本研究旨在通过鉴定具有创新结构和新作用机制的抗真菌药物来应对这一挑战。我们设计合成了一系列用 5-磺酰基-1,3,4-噻二唑取代的类黄酮衍生物,并评价了它们对 5 种植物病原真菌的抗真菌活性。大多数类黄酮衍生物对灰葡萄孢菌 (B. cinerea)、茄链孢菌 (A. solani)、立枯根瘤菌 (R. solani)、禾谷镰刀菌 (F. graminearum) 和禾谷炭疽菌 (C. orbiculare) 表现出优异的抗真菌活性。具体而言,38 种目标化合物对立枯菌的 EC50 值均低于 4 μg/mL,其中化合物 C13 (EC50 = 0.49 μg/mL)、C15 (EC50 = 0.37 μg/mL) 和 C19 (EC50 = 0.37 μg/mL) 具有最显著的抗真菌活性,优于对照药物多菌灵 (EC50= 0.52 μg/mL)。用化合物 C19 处理后,立枯菌丝体和细胞的细胞超微结构的扫描电子显微镜 (SEM) 和透射电子显微镜 (TEM) 图像显示菌丝的蔓延生长、细胞壁轮廓扭曲和线粒体数量减少。 研究 5-磺酰基-1,3,4-噻二唑取代的类黄酮衍生物的分子结构与抗真菌活性之间的 3D-QSAR 可以显着改善常规药物分子设计途径,并促进新型抗真菌先导化合物的开发。
更新日期:2024-09-17
中文翻译:

新型 5-磺酰基-1,3,4-噻二唑类黄酮的设计、合成、抗真菌评价和三维定量构效关系
植物病原真菌经常破坏植物的正常生理生化功能,导致疾病,损害植物健康,最终降低作物产量。本研究旨在通过鉴定具有创新结构和新作用机制的抗真菌药物来应对这一挑战。我们设计合成了一系列用 5-磺酰基-1,3,4-噻二唑取代的类黄酮衍生物,并评价了它们对 5 种植物病原真菌的抗真菌活性。大多数类黄酮衍生物对灰葡萄孢菌 (B. cinerea)、茄链孢菌 (A. solani)、立枯根瘤菌 (R. solani)、禾谷镰刀菌 (F. graminearum) 和禾谷炭疽菌 (C. orbiculare) 表现出优异的抗真菌活性。具体而言,38 种目标化合物对立枯菌的 EC50 值均低于 4 μg/mL,其中化合物 C13 (EC50 = 0.49 μg/mL)、C15 (EC50 = 0.37 μg/mL) 和 C19 (EC50 = 0.37 μg/mL) 具有最显著的抗真菌活性,优于对照药物多菌灵 (EC50= 0.52 μg/mL)。用化合物 C19 处理后,立枯菌丝体和细胞的细胞超微结构的扫描电子显微镜 (SEM) 和透射电子显微镜 (TEM) 图像显示菌丝的蔓延生长、细胞壁轮廓扭曲和线粒体数量减少。 研究 5-磺酰基-1,3,4-噻二唑取代的类黄酮衍生物的分子结构与抗真菌活性之间的 3D-QSAR 可以显着改善常规药物分子设计途径,并促进新型抗真菌先导化合物的开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号