当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Bioactivity, and Action Mechanism of Pyridinecarbaldehyde Phenylhydrazone Derivatives with Broad-Spectrum Antifungal Activity
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-17 , DOI: 10.1021/acs.jafc.4c04078 Bohang Zhou 1, 2 , Juan Fu 3 , Yuhao Zhang 3 , Ruofei Bai 3 , Yiwei Wang 3 , Yiwei Yang 1, 2 , Yingmei Li 1, 2 , Le Zhou 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-17 , DOI: 10.1021/acs.jafc.4c04078 Bohang Zhou 1, 2 , Juan Fu 3 , Yuhao Zhang 3 , Ruofei Bai 3 , Yiwei Wang 3 , Yiwei Yang 1, 2 , Yingmei Li 1, 2 , Le Zhou 3
Affiliation
|
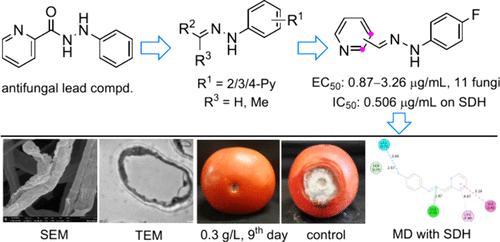
Replacing old pesticides with new pesticide varieties has been the main means to solve pesticide resistance. Therefore, it is necessary to research and develop new antifungal agents for plant protection. In this study, a series of pyridinecarbaldehyde phenylhydrazone derivatives were designed and evaluated for their inhibition activity on plant pathogenic fungi to search for novel fungicide candidates. Picolinaldehyde phenylhydrazone (1) and nicotinaldehyde phenylhydrazone (2) were identified as promising antifungal lead scaffolds. The 4-fluorophenylhydrazone derivatives (1a and 2a) of 1 and 2 showed highly effective and broad-spectrum inhibition activity in vitro on 11 phytopathogenic fungi with EC50 values of 0.870–3.26 μg/mL, superior to the positive control carbendazim in most cases. The presence of the 4-fluorine atom on the phenyl showed a remarkable activity enhancement effect. Compound 1a at 300 μg/mL provided almost complete protection against infection of Alternaria solani on tomatoes over the post-treatment 9 days and high safety to germination of plant seeds. Furthermore, 1a showed strong inhibition activity with an IC50 value of 0.506 μg/mL on succinate dehydrogenase in A. solani. Molecular docking showed that both 1a and 2a can well bind to the ubiquinone-binding region of SDH by the conventional hydrogen bond, carbon–hydrogen bond, π–π or π–amide interaction, π–alkyl interaction, X---F (X = N, C, or H) interaction, and van der Waal forces. Meanwhile, scanning and transmission electron analysis displayed that 1a destroyed the morphology of mycelium and the structure of the cell membrane of A. solani. Fluorescent staining analysis revealed that 1a changed the mitochondrial membrane potential and cell membrane permeability. Thus, pyridinecarbaldehyde phenylhydrazone compounds emerged as novel antifungal lead scaffolds, and 1a and 2a can be considered promising candidates for the development of new agricultural fungicides.
中文翻译:

广谱抗真菌吡啶甲醛苯腙衍生物的设计、生物活性及作用机制
用新农药品种替代旧农药已成为解决农药抗性的主要手段。因此,有必要研究和开发新型植物保护抗真菌剂。本研究设计了一系列吡啶甲醛苯腙衍生物,并评估其对植物病原真菌的抑制活性,以寻找新型杀菌剂候选物。吡啶甲醛苯腙 ( 1 ) 和烟醛苯腙 ( 2 ) 被认为是有前景的抗真菌铅支架。 1和2的4-氟苯腙衍生物( 1a和2a )对11种植物病原真菌具有高效、广谱的体外抑制活性,EC 50值为0.870~3.26 μg/mL,大多数情况优于阳性对照多菌灵。 。苯基上4-氟原子的存在表现出显着的活性增强效果。 300 μg/mL 的化合物1a在处理后 9 天期间几乎完全防止番茄上的链格孢菌感染,并且对植物种子的发芽具有高度安全性。此外, 1a对茄病菌琥珀酸脱氢酶表现出较强的抑制活性,IC 50值为0.506 μg/mL。分子对接表明1a和2a均能通过常规氢键、碳氢键、π-π或π-酰胺相互作用、π-烷基相互作用、X---F等与SDH的泛醌结合区良好结合。 X = N、C 或 H) 相互作用和范德华力。 同时,扫描和透射电子分析表明, 1a破坏了茄病菌菌丝体的形态和细胞膜的结构。荧光染色分析显示1a改变了线粒体膜电位和细胞膜通透性。因此,吡啶甲醛苯腙化合物作为新型抗真菌先导支架出现, 1a和2a可以被认为是开发新型农业杀菌剂的有希望的候选者。
更新日期:2024-09-17
中文翻译:

广谱抗真菌吡啶甲醛苯腙衍生物的设计、生物活性及作用机制
用新农药品种替代旧农药已成为解决农药抗性的主要手段。因此,有必要研究和开发新型植物保护抗真菌剂。本研究设计了一系列吡啶甲醛苯腙衍生物,并评估其对植物病原真菌的抑制活性,以寻找新型杀菌剂候选物。吡啶甲醛苯腙 ( 1 ) 和烟醛苯腙 ( 2 ) 被认为是有前景的抗真菌铅支架。 1和2的4-氟苯腙衍生物( 1a和2a )对11种植物病原真菌具有高效、广谱的体外抑制活性,EC 50值为0.870~3.26 μg/mL,大多数情况优于阳性对照多菌灵。 。苯基上4-氟原子的存在表现出显着的活性增强效果。 300 μg/mL 的化合物1a在处理后 9 天期间几乎完全防止番茄上的链格孢菌感染,并且对植物种子的发芽具有高度安全性。此外, 1a对茄病菌琥珀酸脱氢酶表现出较强的抑制活性,IC 50值为0.506 μg/mL。分子对接表明1a和2a均能通过常规氢键、碳氢键、π-π或π-酰胺相互作用、π-烷基相互作用、X---F等与SDH的泛醌结合区良好结合。 X = N、C 或 H) 相互作用和范德华力。 同时,扫描和透射电子分析表明, 1a破坏了茄病菌菌丝体的形态和细胞膜的结构。荧光染色分析显示1a改变了线粒体膜电位和细胞膜通透性。因此,吡啶甲醛苯腙化合物作为新型抗真菌先导支架出现, 1a和2a可以被认为是开发新型农业杀菌剂的有希望的候选者。

































 京公网安备 11010802027423号
京公网安备 11010802027423号