当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toward Methanol Production by CO2 Hydrogenation beyond Formic Acid Formation
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.accounts.4c00411 Naoya Onishi 1 , Yuichiro Himeda 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.accounts.4c00411 Naoya Onishi 1 , Yuichiro Himeda 1, 2
Affiliation
|
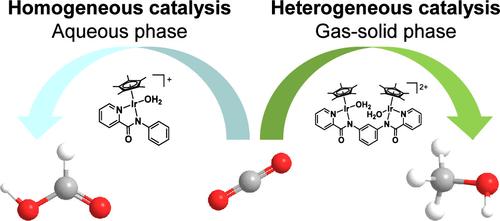
The Paradigm shift in considering CO2 as an alternative carbon feedstock as opposed to a waste product has recently prompted intense research activities. The implementation of CO2 utilization may be achieved by designing highly efficient catalysts, exploring processes that minimize energy consumption and simplifying product purification and separation. Among possible target products derived from CO2, methanol is highly valuable because it can be used in various chemical feedstocks and as a fuel. Although it is currently produced on a plant scale by heterogeneous catalysis using a Cu/ZnO-based catalyst, a limited theoretical conversion ratio at high reaction temperatures remains an issue. In addition, a catalytic system that can be adjusted to accommodate a variable renewable energy source for the synthesis of methanol is more desirable than current continuous-operation systems, which require a reliable energy supply. Recently, significant progress has been made in the field of homogeneous catalysis, which primarily relies on an indirect route to synthesize methanol via the hydrogenation of carbonate or formate derivatives in the presence of additives and solvents. However, homogeneous catalysis is inappropriate for industrial-scale methanol production because of the inefficient separation and purification processes involved.
中文翻译:

超越甲酸形成的二氧化碳加氢生产甲醇
将CO 2视为替代碳原料而不是废物的范式转变最近引发了激烈的研究活动。 CO 2利用的实施可以通过设计高效催化剂、探索最小化能源消耗的工艺以及简化产物纯化和分离来实现。在源自CO 2的可能目标产品中,甲醇具有很高的价值,因为它可用于各种化学原料和作为燃料。尽管目前它是使用 Cu/ZnO 基催化剂通过多相催化在工厂规模生产的,但在高反应温度下有限的理论转化率仍然是一个问题。此外,可以调整以适应用于合成甲醇的可变可再生能源的催化系统比当前需要可靠的能源供应的连续运行系统更理想。近年来,均相催化领域取得了重大进展,该领域主要依靠在添加剂和溶剂存在下通过碳酸酯或甲酸酯衍生物的加氢间接途径合成甲醇。然而,均相催化由于涉及低效的分离和纯化过程,不适合工业规模的甲醇生产。
更新日期:2024-09-16
中文翻译:

超越甲酸形成的二氧化碳加氢生产甲醇
将CO 2视为替代碳原料而不是废物的范式转变最近引发了激烈的研究活动。 CO 2利用的实施可以通过设计高效催化剂、探索最小化能源消耗的工艺以及简化产物纯化和分离来实现。在源自CO 2的可能目标产品中,甲醇具有很高的价值,因为它可用于各种化学原料和作为燃料。尽管目前它是使用 Cu/ZnO 基催化剂通过多相催化在工厂规模生产的,但在高反应温度下有限的理论转化率仍然是一个问题。此外,可以调整以适应用于合成甲醇的可变可再生能源的催化系统比当前需要可靠的能源供应的连续运行系统更理想。近年来,均相催化领域取得了重大进展,该领域主要依靠在添加剂和溶剂存在下通过碳酸酯或甲酸酯衍生物的加氢间接途径合成甲醇。然而,均相催化由于涉及低效的分离和纯化过程,不适合工业规模的甲醇生产。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号