Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SlipO2Chip – single-cell respiration under tuneable environments
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-18 , DOI: 10.1039/d4lc00420e Yuan Cui 1 , Milena De Albuquerque Moreira 2 , Kristen E Whalen 3 , Laurent Barbe 4 , Qian Shi 4 , Klaus Koren 5 , Maria Tenje 4 , Lars Behrendt 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-18 , DOI: 10.1039/d4lc00420e Yuan Cui 1 , Milena De Albuquerque Moreira 2 , Kristen E Whalen 3 , Laurent Barbe 4 , Qian Shi 4 , Klaus Koren 5 , Maria Tenje 4 , Lars Behrendt 1
Affiliation
|
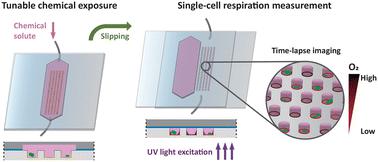
In disciplines like toxicology and pharmacology, oxygen (O2) respiration is a universal metric for evaluating the effects of chemicals across various model systems, including mammalian and microalgal cells. However, for these cells the common practice is to segregate populations into control and exposure groups, which assumes direct equivalence in their responses and does not take into account heterogeneity among individual cells. This lack of resolution impedes our ability to precisely investigate differences among experimental groups with small or limited sample sizes. To overcome this barrier, we introduce SlipO2Chip, an innovative glass microfluidic platform for precisely quantifying single-cell O2 respiration in the coordinated absence and presence of chemical solutes. SlipO2Chip comprises a wet-etched fused silica channel plate on the top and a dry-etched borosilicate microwell plate at the bottom. The microwells are coated with Pt(II) meso-tetra(pentafluorophenyl)porphine (PtTFPP), an O2 sensing optode material and an O2-independent reference dye. A custom 3D-printed holder facilitates the controlled horizontal movement (‘slipping’) of the channel plate over the microwell plate, thereby establishing or disrupting the fluid path over microwells. Collectively, these design elements enable the immobilization of single-cells in microwells, their exposure to controlled fluid flows, the coordinated opening and closing of microwells and repeated measurements of single-cell O2 respiration. Uniquely, by sequentially executing opening and closing it becomes possible to measure single-cell respiration prior to and after exposure to chemical solutes. In a proof-of-concept application, we utilized SlipO2Chip to measure the impact of increasing exposures of the marine bacterial signal 2-heptyl-4-quinolone (HHQ) on the dark respiration of the diatom Ditylum brightwellii at single-cell resolution. Results revealed a concentration-dependent decrease in per-cell O2 dark respiration, with a maximum reduction of 40.2% observed at HHQ concentrations exceeding 35.5 μM, and a half-maximal effective concentration (EC50) of 5.8 μM, consistent with that obtained via conventional bulk respiration methods. The ability of SlipO2Chip to sequentially assess the effects of chemical substances on single-cell O2 metabolism is advantageous for research where sample volumes are limited, such as clinical biopsies, studies involving rare microbial isolates, and toxicological studies aiming to address exposure effects while accounting for cell-to-cell variability.
中文翻译:

SlipO2Chip – 可调环境下的单细胞呼吸
在毒理学和药理学等学科中,氧气 (O2) 呼吸是评估化学品对各种模型系统(包括哺乳动物和微藻细胞)影响的通用指标。然而,对于这些细胞,通常的做法是将群体分为对照组和暴露组,这假设它们的反应直接等效,并且没有考虑单个细胞之间的异质性。这种分辨率的缺乏阻碍了我们精确研究样本量小或有限的实验组之间的差异的能力。为了克服这一障碍,我们推出了 SlipO2Chip,这是一种创新的玻璃微流控平台,用于在化学溶质协调缺失和存在的情况下精确量化单细胞 O2 呼吸。SlipO2芯片包括顶部的湿法蚀刻熔融石英通道板和底部的干法蚀刻硼硅酸盐微孔板。微孔包被有 Pt(II) 内消旋四(五氟苯基)卟啉 (PtTFPP)、O2 传感光材料和 O2 非依赖性参比染料。定制的 3D 打印支架有助于通道板在微孔板上的受控水平移动(“滑动”),从而建立或破坏微孔上的流体路径。总的来说,这些设计元素能够将单细胞固定在微孔中,使其暴露在受控的流体流动中,协调打开和关闭微孔以及重复测量单细胞 O2 呼吸。 独特的是,通过依次执行打开和关闭,可以测量暴露于化学溶质之前和之后的单细胞呼吸。在概念验证应用中,我们利用 SlipO2芯片在单细胞分辨率下测量海洋细菌信号 2-庚基-4-喹诺酮 (HHQ) 暴露增加对硅藻 Ditylum brightwellii 暗呼吸的影响。结果显示,每细胞 O2 暗呼吸呈浓度依赖性降低,在 HHQ 浓度超过 35.5 μM 时观察到最大降低 40.2%,半数最大有效浓度 (EC50) 为 5.8 μM,与通过常规批量呼吸方法获得的结果一致。SlipO2Chip 能够按顺序评估化学物质对单细胞 O2 代谢的影响,这对于样本量有限的研究是有利的,例如临床活检、涉及稀有微生物分离物的研究以及旨在解决暴露效应同时考虑细胞间变异性的毒理学研究。
更新日期:2024-09-18
中文翻译:

SlipO2Chip – 可调环境下的单细胞呼吸
在毒理学和药理学等学科中,氧气 (O2) 呼吸是评估化学品对各种模型系统(包括哺乳动物和微藻细胞)影响的通用指标。然而,对于这些细胞,通常的做法是将群体分为对照组和暴露组,这假设它们的反应直接等效,并且没有考虑单个细胞之间的异质性。这种分辨率的缺乏阻碍了我们精确研究样本量小或有限的实验组之间的差异的能力。为了克服这一障碍,我们推出了 SlipO2Chip,这是一种创新的玻璃微流控平台,用于在化学溶质协调缺失和存在的情况下精确量化单细胞 O2 呼吸。SlipO2芯片包括顶部的湿法蚀刻熔融石英通道板和底部的干法蚀刻硼硅酸盐微孔板。微孔包被有 Pt(II) 内消旋四(五氟苯基)卟啉 (PtTFPP)、O2 传感光材料和 O2 非依赖性参比染料。定制的 3D 打印支架有助于通道板在微孔板上的受控水平移动(“滑动”),从而建立或破坏微孔上的流体路径。总的来说,这些设计元素能够将单细胞固定在微孔中,使其暴露在受控的流体流动中,协调打开和关闭微孔以及重复测量单细胞 O2 呼吸。 独特的是,通过依次执行打开和关闭,可以测量暴露于化学溶质之前和之后的单细胞呼吸。在概念验证应用中,我们利用 SlipO2芯片在单细胞分辨率下测量海洋细菌信号 2-庚基-4-喹诺酮 (HHQ) 暴露增加对硅藻 Ditylum brightwellii 暗呼吸的影响。结果显示,每细胞 O2 暗呼吸呈浓度依赖性降低,在 HHQ 浓度超过 35.5 μM 时观察到最大降低 40.2%,半数最大有效浓度 (EC50) 为 5.8 μM,与通过常规批量呼吸方法获得的结果一致。SlipO2Chip 能够按顺序评估化学物质对单细胞 O2 代谢的影响,这对于样本量有限的研究是有利的,例如临床活检、涉及稀有微生物分离物的研究以及旨在解决暴露效应同时考虑细胞间变异性的毒理学研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号