当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Cation Activation of N-Enriched Porous Carbons Improves Control of CO2 and N2 Adsorption Thermodynamics for Selective CO2 Capture
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-17 , DOI: 10.1002/adfm.202410171 J. Ehren Eichler 1 , Hanah Leonard 2 , Ethan Kang Yang 1 , Lettie A. Smith 1 , Samantha N. Lauro 1 , James N. Burrow 3 , Rui P. P. L. Ribeiro 4, 5 , C. Buddie Mullins 1, 3
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-17 , DOI: 10.1002/adfm.202410171 J. Ehren Eichler 1 , Hanah Leonard 2 , Ethan Kang Yang 1 , Lettie A. Smith 1 , Samantha N. Lauro 1 , James N. Burrow 3 , Rui P. P. L. Ribeiro 4, 5 , C. Buddie Mullins 1, 3
Affiliation
|
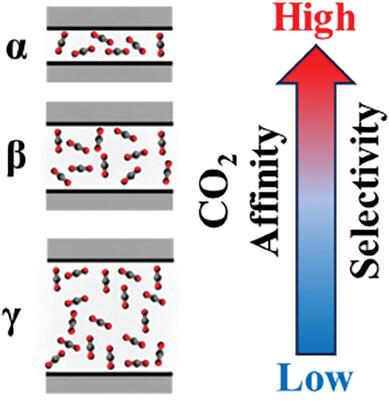
Porous carbons have potential to facilitate energy-efficient separation of CO2 from post-combustion flue gas. However, the complicated interplay between chemical and textural properties has prevented a comprehensive understanding of selective CO2 adsorption. This study demonstrates how dual cation activation of carbons serves as a synthetic platform to help modulate porosity independent of nitrogen content. For samples derived from nitrogen-poor precursors, surface areas deviated significantly (2200–4500 m2 g−1) at a constant total nitrogen content (2.3 ± 0.3 at %). Surface area changed less for samples derived from nitrogen-rich precursors (400–675 m2 g−1 at 23.1 ± 0.1 at % N). Rigorous structure-function and thermodynamic analysis of these carbons not only helped to uncover the nature of the different adsorption sites, but also established a fundamental linear free energy exchange relationship. This coupled with material property correlations informed the properties that facilitated selective capture of CO2. Critically, for these physisorptive carbons, selectivity is almost entirely a function of relative porosity and chemical adsorbent-adsorbate interactions play a negligible role.
中文翻译:

富氮多孔碳的双阳离子活化改善了对 CO2 和 N2 吸附热力学的控制,用于选择性 CO2 捕获
多孔碳具有促进从燃烧后烟气中节能分离 CO2 的潜力。然而,化学性质和质构性质之间复杂的相互作用阻碍了对选择性 CO2 吸附的全面理解。本研究展示了碳的双重阳离子活化如何作为合成平台来帮助调节独立于氮含量的孔隙率。对于来自贫氮前体的样品,在总氮含量恒定(2.3 ± 0.3 % 时)下,表面积显著偏差 (2200–4500 m2 g-1)。来自富氮前体的样品的表面积变化较小(400-675 m2 g-1,23.1 ± 0.1,% N 时)。对这些碳的严格结构函数和热力学分析不仅有助于揭示不同吸附位点的性质,而且还建立了基本的线性自由能交换关系。这与材料特性相关性相结合,为促进 CO2 选择性捕获的特性提供了信息。至关重要的是,对于这些物理吸附碳,选择性几乎完全是相对孔隙率的函数,化学吸附剂-吸附物相互作用的作用可以忽略不计。
更新日期:2024-09-17
中文翻译:

富氮多孔碳的双阳离子活化改善了对 CO2 和 N2 吸附热力学的控制,用于选择性 CO2 捕获
多孔碳具有促进从燃烧后烟气中节能分离 CO2 的潜力。然而,化学性质和质构性质之间复杂的相互作用阻碍了对选择性 CO2 吸附的全面理解。本研究展示了碳的双重阳离子活化如何作为合成平台来帮助调节独立于氮含量的孔隙率。对于来自贫氮前体的样品,在总氮含量恒定(2.3 ± 0.3 % 时)下,表面积显著偏差 (2200–4500 m2 g-1)。来自富氮前体的样品的表面积变化较小(400-675 m2 g-1,23.1 ± 0.1,% N 时)。对这些碳的严格结构函数和热力学分析不仅有助于揭示不同吸附位点的性质,而且还建立了基本的线性自由能交换关系。这与材料特性相关性相结合,为促进 CO2 选择性捕获的特性提供了信息。至关重要的是,对于这些物理吸附碳,选择性几乎完全是相对孔隙率的函数,化学吸附剂-吸附物相互作用的作用可以忽略不计。































 京公网安备 11010802027423号
京公网安备 11010802027423号