当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxadiazolines as Photoreleasable Labels for Drug Target Identification
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c06936 Corentin Bon 1 , Benedikt Goretzki 2 , Marie Flamme 3 , Claude Shelton 4 , Holly Davis 1 , Fabio Lima 1 , Francisco Garcia 4 , Scott Brittain 4 , Cara E Brocklehurst 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c06936 Corentin Bon 1 , Benedikt Goretzki 2 , Marie Flamme 3 , Claude Shelton 4 , Holly Davis 1 , Fabio Lima 1 , Francisco Garcia 4 , Scott Brittain 4 , Cara E Brocklehurst 1
Affiliation
|
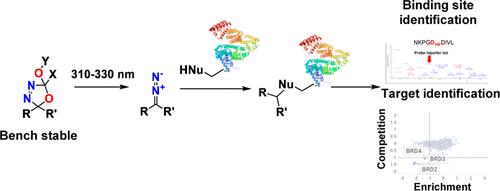
Photoaffinity labeling is a widely used technique for studying ligand–protein and protein–protein interactions. Traditional photoaffinity labels utilize nonspecific C–H bond insertion reactions mediated by a highly reactive intermediate. Despite being the most widely used photoaffinity labels, diazirines exhibit limited compatibility with downstream organic reactions and suffer from storage stability concerns. This study introduces oxadiazolines as innovative and complementary photoactivatable labels for addition to the toolbox and demonstrates their application in vitro and through in cellulo labeling experiments. Oxadiazolines can be easily synthesized from ketone moieties and cleaved with 302–330 nm light to cleanly liberate a diazo reactive moiety that can covalently modify nucleophilic amino acid residues. Notably, oxadiazolines are compatible with various organic reaction conditions and functional groups, allowing for the exploration of a large chemical space. Several known inhibitors featuring the oxadiazoline functionality were prepared without affecting their binding affinity. Furthermore, we confirmed the ability of oxadiazolines to form covalent bonds with proteins upon UV-irradiation, both in vitro and in cellulo, yielding comparable results to those of the matched diazirine compounds.
中文翻译:

恶二唑啉作为用于药物靶标鉴定的光可释放标记
光亲和标记是研究配体-蛋白质和蛋白质-蛋白质相互作用的一种广泛使用的技术。传统的光亲和标记利用由高反应性中间体介导的非特异性 C-H 键插入反应。尽管二嗪嗪是应用最广泛的光亲和标记物,但与下游有机反应的相容性有限,并且存在储存稳定性问题。本研究将恶二唑啉作为创新和互补的光激活标记物引入工具箱,并展示了它们在体外和赛璐鲁罗标记实验中的应用。恶二唑啉可以很容易地从酮部分合成,并用 302-330 nm 光裂解,以干净地释放出可以共价修饰亲核氨基酸残基的重氮反应性部分。值得注意的是,恶二唑啉与各种有机反应条件和官能团相容,允许探索较大的化学空间。制备了几种具有恶二唑啉官能团的已知抑制剂,而不会影响它们的结合亲和力。此外,我们证实了恶二唑啉在紫外线照射下在体外和纤维素中与蛋白质形成共价键的能力,产生了与匹配的二氮嗪化合物相当的结果。
更新日期:2024-09-17
中文翻译:

恶二唑啉作为用于药物靶标鉴定的光可释放标记
光亲和标记是研究配体-蛋白质和蛋白质-蛋白质相互作用的一种广泛使用的技术。传统的光亲和标记利用由高反应性中间体介导的非特异性 C-H 键插入反应。尽管二嗪嗪是应用最广泛的光亲和标记物,但与下游有机反应的相容性有限,并且存在储存稳定性问题。本研究将恶二唑啉作为创新和互补的光激活标记物引入工具箱,并展示了它们在体外和赛璐鲁罗标记实验中的应用。恶二唑啉可以很容易地从酮部分合成,并用 302-330 nm 光裂解,以干净地释放出可以共价修饰亲核氨基酸残基的重氮反应性部分。值得注意的是,恶二唑啉与各种有机反应条件和官能团相容,允许探索较大的化学空间。制备了几种具有恶二唑啉官能团的已知抑制剂,而不会影响它们的结合亲和力。此外,我们证实了恶二唑啉在紫外线照射下在体外和纤维素中与蛋白质形成共价键的能力,产生了与匹配的二氮嗪化合物相当的结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号