当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Decarboxylative C(sp3)-C(sp3) Cross-Coupling of Aliphatic Redox-Active Esters with gem-Borazirconocene Alkanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c09245 Jing Wang 1, 2, 3 , Songlin Bai 1, 2, 3 , Chao Yang 4 , Xiangbing Qi 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c09245 Jing Wang 1, 2, 3 , Songlin Bai 1, 2, 3 , Chao Yang 4 , Xiangbing Qi 2, 3
Affiliation
|
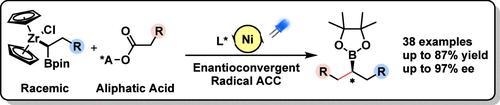
Asymmetric decarboxylative cross-couplings of carboxylic acids are powerful methods for synthesizing chiral building blocks essential in medicinal chemistry and material science. Despite their potential, creating versatile chiral alkylboron derivatives through asymmetric decarboxylative C(sp3)-C(sp3) cross-coupling from readily available primary aliphatic acids and mild organometallic reagents remains challenging. In this study, we present a visible light-induced Ni-catalyzed enantioconvergent C(sp3)-C(sp3) cross-coupling of unactivated primary aliphatic acid NHPI esters with gem-borazirconocene alkanes, producing a diverse array of valuable chiral alkylboron building blocks. The method boasts a broad substrate scope, high functional group tolerance, and the ability for late-stage modification of complex drug molecules and natural products with high enantioselectivity, showcasing its synthetic potential. Mechanistic investigations suggest a nickel-catalyzed enantioconvergent radical cross-coupling pathway, wherein the primary radical from a redox-active ester is generated through single-electron reduction with ZrIII species. This represents an unprecedented example of enantioselective radical C(sp3)-C(sp3) cross-coupling in the absence of photocatalysts.
中文翻译:

脂肪族氧化还原活性酯与 gem-Borazirconocene 烷烃的对映选择性脱羧 C(sp3)-C(sp3) 交叉偶联
羧酸的不对称脱羧交叉偶联是合成药物化学和材料科学中必不可少的手性结构单元的强大方法。尽管它们具有潜力,但通过现成的伯脂肪酸和温和有机金属试剂的不对称脱羧 C(sp3)-C(sp3) 交叉偶联产生多功能手性烷基硼衍生物仍然具有挑战性。在这项研究中,我们提出了一种可见光诱导的镍催化的对映趋同 C(sp3)-C(sp3) 未活化的伯脂肪酸 NHPI 酯与 gem-硼硼烯烷烃的交叉偶联,产生了多种有价值的手性烷基硼结构单元。该方法具有底物范围广、官能团耐受性高、对复杂药物分子和高对映选择性天然产物的后期修饰能力,展现了其合成潜力。机理研究表明,镍催化的对映趋同自由基交叉偶联途径,其中氧化还原活性酯的伯自由基是通过 ZrIII 物质的单电子还原产生的。这代表了在没有光催化剂的情况下对映选择性自由基 C(sp3)-C(sp3) 交叉偶联的前所未有的例子。
更新日期:2024-09-17
中文翻译:

脂肪族氧化还原活性酯与 gem-Borazirconocene 烷烃的对映选择性脱羧 C(sp3)-C(sp3) 交叉偶联
羧酸的不对称脱羧交叉偶联是合成药物化学和材料科学中必不可少的手性结构单元的强大方法。尽管它们具有潜力,但通过现成的伯脂肪酸和温和有机金属试剂的不对称脱羧 C(sp3)-C(sp3) 交叉偶联产生多功能手性烷基硼衍生物仍然具有挑战性。在这项研究中,我们提出了一种可见光诱导的镍催化的对映趋同 C(sp3)-C(sp3) 未活化的伯脂肪酸 NHPI 酯与 gem-硼硼烯烷烃的交叉偶联,产生了多种有价值的手性烷基硼结构单元。该方法具有底物范围广、官能团耐受性高、对复杂药物分子和高对映选择性天然产物的后期修饰能力,展现了其合成潜力。机理研究表明,镍催化的对映趋同自由基交叉偶联途径,其中氧化还原活性酯的伯自由基是通过 ZrIII 物质的单电子还原产生的。这代表了在没有光催化剂的情况下对映选择性自由基 C(sp3)-C(sp3) 交叉偶联的前所未有的例子。































 京公网安备 11010802027423号
京公网安备 11010802027423号