当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Deactivation-Resistant Dialkylbiarylphosphine Ligand for Pd-Catalyzed Arylation of Secondary Amines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c09667 Kaibo Feng 1 , Elaine Reichert Raguram 1 , James R Howard 2 , Ellyn Peters 2 , Cecilia Liu 1 , Matthew S Sigman 2 , Stephen L Buchwald 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c09667 Kaibo Feng 1 , Elaine Reichert Raguram 1 , James R Howard 2 , Ellyn Peters 2 , Cecilia Liu 1 , Matthew S Sigman 2 , Stephen L Buchwald 1
Affiliation
|
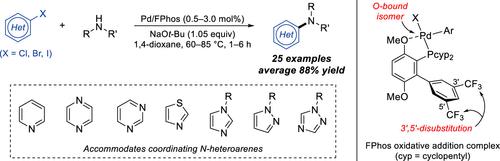
Despite the prevalence of N-heteroarenes in small-molecule pharmaceuticals, Pd-catalyzed C–N cross-coupling reactions of aryl halides and amines containing these rings remain challenging due to their ability to displace the supporting ligand via coordination to the metal center. To address this limitation, we report the development of a highly robust Pd catalyst supported by a new dialkylbiarylphosphine ligand, FPhos. The FPhos-supported catalyst effectively resists N-heteroarene-mediated catalyst deactivation to readily promote C–N coupling between a wide variety of Lewis-basic aryl halides and secondary amines, including densely functionalized pharmaceuticals. Mechanistic and structural investigations, as well as principal component analysis and density functional theory, elucidated two key design features that enable FPhos to overcome the limitations of previous ligands. First, the ligated Pd complex is stabilized through its conformational preference for the O-bound isomer, which likely resists coordination by N-heteroarenes. Second, 3′,5′-disubstitution on the non-phosphorus-containing ring of FPhos creates the ideal steric environment around the Pd center, which facilitates binding by larger secondary amines while mitigating the formation of off-cycle palladacycle species.
中文翻译:

开发用于 Pd 催化仲胺芳基化的抗失活二烷基二芳基膦配体
尽管N-杂芳烃在小分子药物中普遍存在,但钯催化的芳基卤化物和含有这些环的胺的C-N交叉偶联反应仍然具有挑战性,因为它们能够通过与金属中心的配位来取代支持配体。为了解决这一限制,我们报告了一种由新型二烷基联芳基膦配体 FPhos 支持的高度稳健的 Pd 催化剂的开发。 FPhos负载的催化剂有效地抵抗N-杂芳烃介导的催化剂失活,从而容易促进各种路易斯碱性芳基卤化物和仲胺(包括密集功能化药物)之间的C-N偶联。机理和结构研究以及主成分分析和密度泛函理论阐明了 FPhos 能够克服先前配体局限性的两个关键设计特征。首先,连接的 Pd 络合物通过其对 O 结合异构体的构象偏好而稳定,这可能会抵抗 N-杂芳烃的配位。其次,FPhos 不含磷的环上的 3',5'-二取代在 Pd 中心周围创造了理想的空间环境,有利于较大仲胺的结合,同时减轻非循环钯环物质的形成。
更新日期:2024-09-17
中文翻译:

开发用于 Pd 催化仲胺芳基化的抗失活二烷基二芳基膦配体
尽管N-杂芳烃在小分子药物中普遍存在,但钯催化的芳基卤化物和含有这些环的胺的C-N交叉偶联反应仍然具有挑战性,因为它们能够通过与金属中心的配位来取代支持配体。为了解决这一限制,我们报告了一种由新型二烷基联芳基膦配体 FPhos 支持的高度稳健的 Pd 催化剂的开发。 FPhos负载的催化剂有效地抵抗N-杂芳烃介导的催化剂失活,从而容易促进各种路易斯碱性芳基卤化物和仲胺(包括密集功能化药物)之间的C-N偶联。机理和结构研究以及主成分分析和密度泛函理论阐明了 FPhos 能够克服先前配体局限性的两个关键设计特征。首先,连接的 Pd 络合物通过其对 O 结合异构体的构象偏好而稳定,这可能会抵抗 N-杂芳烃的配位。其次,FPhos 不含磷的环上的 3',5'-二取代在 Pd 中心周围创造了理想的空间环境,有利于较大仲胺的结合,同时减轻非循环钯环物质的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号