当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoexcited Copper-Catalyzed Enantioselective Allylic C(sp3)–H Acyloxylation of Acyclic Internal Alkenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c11145 Sheng Tang 1 , Hui Xu 2 , Yanfeng Dang 2 , Shouyun Yu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c11145 Sheng Tang 1 , Hui Xu 2 , Yanfeng Dang 2 , Shouyun Yu 1
Affiliation
|
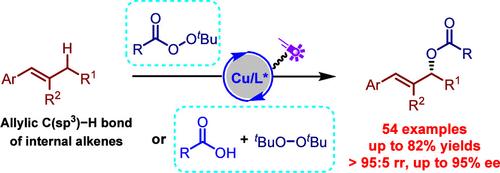
The functionalization of C–H bonds streamlines the synthesis of complex molecules by eliminating the need for substrate preactivation. Traditionally, the Kharasch–Sosnovsky reaction, which directly oxidizes allylic C–H bonds into allylic esters under copper catalysis, has been hampered by long reaction times, limited substrate scope, and low enantioselectivity with acyclic olefins. Herein, we present a novel, visible light-driven, copper-catalyzed asymmetric Kharasch–Sosnovsky reaction that overcomes these challenges. This method expands the substrate scope to include acyclic internal alkenes and improves reaction conditions using eco-friendly visible light catalysis. It enhances radical reactivity and achieves superior enantioselectivity and regioselectivity in producing allylic C–H acyloxylation products. This breakthrough significantly advances direct C–H functionalization techniques, offering a more efficient and sustainable approach to synthesizing chiral molecules.
中文翻译:

光激发铜催化无环内烯烃的对映选择性烯丙基 C(sp3)–H 酰氧基化
C-H 键的功能化无需底物预激活,从而简化了复杂分子的合成。传统上,Kharasch-Sosnovsky 反应在铜催化下直接将烯丙型 C-H 键氧化成烯丙酯,但由于反应时间长、底物范围有限以及与无环烯烃的对映选择性低而受到阻碍。在此,我们提出了一种新颖的、可见光驱动的、铜催化的不对称卡拉什-索斯诺夫斯基反应,克服了这些挑战。该方法扩大了底物范围,包括无环内烯烃,并利用环保的可见光催化改善了反应条件。它增强了自由基反应性,并在生产烯丙基 C-H 酰氧基化产物时实现了优异的对映选择性和区域选择性。这一突破显着推进了直接 C-H 官能化技术,为合成手性分子提供了一种更有效、更可持续的方法。
更新日期:2024-09-17
中文翻译:

光激发铜催化无环内烯烃的对映选择性烯丙基 C(sp3)–H 酰氧基化
C-H 键的功能化无需底物预激活,从而简化了复杂分子的合成。传统上,Kharasch-Sosnovsky 反应在铜催化下直接将烯丙型 C-H 键氧化成烯丙酯,但由于反应时间长、底物范围有限以及与无环烯烃的对映选择性低而受到阻碍。在此,我们提出了一种新颖的、可见光驱动的、铜催化的不对称卡拉什-索斯诺夫斯基反应,克服了这些挑战。该方法扩大了底物范围,包括无环内烯烃,并利用环保的可见光催化改善了反应条件。它增强了自由基反应性,并在生产烯丙基 C-H 酰氧基化产物时实现了优异的对映选择性和区域选择性。这一突破显着推进了直接 C-H 官能化技术,为合成手性分子提供了一种更有效、更可持续的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号