当前位置:
X-MOL 学术
›
Cell Host Microbe
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microbiota regulates neonatal disease tolerance to virus-evoked necrotizing enterocolitis by shaping the STAT1-NLRC5 axis in the intestinal epithelium
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-09-17 , DOI: 10.1016/j.chom.2024.08.013 Saravanan Subramanian 1 , Hua Geng 1 , Longtao Wu 2 , Chao Du 3 , Amy M Peiper 4 , Heng-Fu Bu 1 , Pauline M Chou 5 , Xiao Wang 1 , Stephanie C Tan 6 , Neha R Iyer 4 , Nazeer Hussain Khan 1 , Ellen L Zechner 7 , James G Fox 8 , Rolf Breinbauer 9 , Chao Qi 5 , Bakhtiar Yamini 2 , Jenny P Ting 10 , Isabelle G De Plaen 3 , Stephanie M Karst 4 , Xiao-Di Tan 11
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-09-17 , DOI: 10.1016/j.chom.2024.08.013 Saravanan Subramanian 1 , Hua Geng 1 , Longtao Wu 2 , Chao Du 3 , Amy M Peiper 4 , Heng-Fu Bu 1 , Pauline M Chou 5 , Xiao Wang 1 , Stephanie C Tan 6 , Neha R Iyer 4 , Nazeer Hussain Khan 1 , Ellen L Zechner 7 , James G Fox 8 , Rolf Breinbauer 9 , Chao Qi 5 , Bakhtiar Yamini 2 , Jenny P Ting 10 , Isabelle G De Plaen 3 , Stephanie M Karst 4 , Xiao-Di Tan 11
Affiliation
|
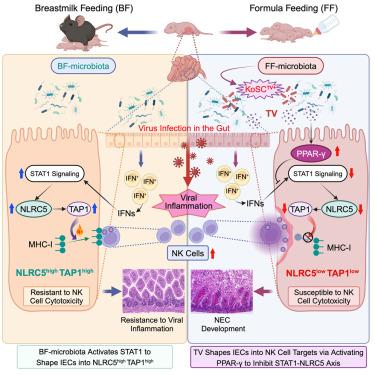
Microbiota and feeding modes influence the susceptibility of premature newborns to necrotizing enterocolitis (NEC) through mechanisms that remain unknown. Here, we show that microbiota colonization facilitated by breastmilk feeding promotes NOD-like receptor family CARD domain containing 5 (Nlrc5) gene expression in mouse intestinal epithelial cells (IECs). Notably, inducible knockout of the Nlrc5 gene in IECs predisposes neonatal mice to NEC-like injury in the small intestine upon viral inflammation in an NK1.1+ cell-dependent manner. By contrast, formula feeding enhances neonatal gut colonization with environment-derived tilivalline-producing Klebsiella spp. Remarkably, tilivalline disrupts microbiota-activated STAT1 signaling that controls Nlrc5 gene expression in IECs through a PPAR-γ-mediated mechanism. Consequently, this dysregulation hinders the resistance of neonatal intestinal epithelium to self-NK1.1+ cell cytotoxicity upon virus infection/colonization, promoting NEC development. Together, we discover the underappreciated role of intestinal microbiota colonization in shaping a disease tolerance program to viral inflammation and elucidate the mechanisms impacting NEC development in neonates.
中文翻译:

微生物群通过塑造肠上皮中的 STAT1-NLRC5 轴来调节新生儿疾病对病毒诱发的坏死性小肠结肠炎的耐受性
微生物群和喂养方式通过尚不清楚的机制影响早产儿对坏死性小肠结肠炎 (NEC) 的易感性。在这里,我们表明母乳喂养促进的微生物群定植促进了小鼠肠上皮细胞 (IEC) 中含有 5 (Nlrc5) 基因的 NOD 样受体家族 CARD 结构域的表达。值得注意的是,IECs 中 Nlrc5 基因的诱导敲除使新生小鼠在病毒炎症时以 NK1.1 + 细胞依赖性方式在小肠中易发生 NEC 样损伤。相比之下,配方奶喂养增强了环境来源的产 tilivalline 的 Klebsiella spp 的新生儿肠道定植。值得注意的是,tilivalline 通过 PPAR 介导的机制破坏了微生物群激活的 STAT1 信号传导,该信号传导控制 IECs 中 Nlrc5 基因表达γ。因此,这种失调阻碍了新生儿肠上皮在病毒感染/定植后对自身 NK1.1 + 细胞毒性的抵抗力,促进了 NEC 的发展。我们一起发现了肠道微生物群定植在塑造病毒炎症的疾病耐受性计划中被低估的作用,并阐明了影响新生儿 NEC 发育的机制。
更新日期:2024-09-17
中文翻译:

微生物群通过塑造肠上皮中的 STAT1-NLRC5 轴来调节新生儿疾病对病毒诱发的坏死性小肠结肠炎的耐受性
微生物群和喂养方式通过尚不清楚的机制影响早产儿对坏死性小肠结肠炎 (NEC) 的易感性。在这里,我们表明母乳喂养促进的微生物群定植促进了小鼠肠上皮细胞 (IEC) 中含有 5 (Nlrc5) 基因的 NOD 样受体家族 CARD 结构域的表达。值得注意的是,IECs 中 Nlrc5 基因的诱导敲除使新生小鼠在病毒炎症时以 NK1.1 + 细胞依赖性方式在小肠中易发生 NEC 样损伤。相比之下,配方奶喂养增强了环境来源的产 tilivalline 的 Klebsiella spp 的新生儿肠道定植。值得注意的是,tilivalline 通过 PPAR 介导的机制破坏了微生物群激活的 STAT1 信号传导,该信号传导控制 IECs 中 Nlrc5 基因表达γ。因此,这种失调阻碍了新生儿肠上皮在病毒感染/定植后对自身 NK1.1 + 细胞毒性的抵抗力,促进了 NEC 的发展。我们一起发现了肠道微生物群定植在塑造病毒炎症的疾病耐受性计划中被低估的作用,并阐明了影响新生儿 NEC 发育的机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号