Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered Bacteriophage-Polymer Nanoassemblies for Treatment of Wound Biofilm Infections
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-17 , DOI: 10.1021/acsnano.4c08671 Jungmi Park 1 , Muhammad Aamir Hassan 1 , Ahmed Nabawy 1 , Cheng Hsuan Li 1 , Mingdi Jiang 1 , Krupa Parmar 2 , Annika Reddivari 1 , Ritabrita Goswami 1 , Taewon Jeon 1 , Robin Patel 2, 3 , Vincent M Rotello 1
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-17 , DOI: 10.1021/acsnano.4c08671 Jungmi Park 1 , Muhammad Aamir Hassan 1 , Ahmed Nabawy 1 , Cheng Hsuan Li 1 , Mingdi Jiang 1 , Krupa Parmar 2 , Annika Reddivari 1 , Ritabrita Goswami 1 , Taewon Jeon 1 , Robin Patel 2, 3 , Vincent M Rotello 1
Affiliation
|
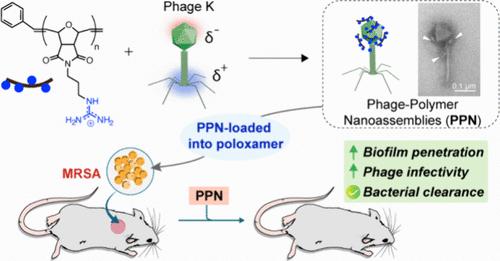
The antibacterial efficacy and specificity of lytic bacteriophages (phages) make them promising therapeutics for treatment of multidrug-resistant bacterial infections. Restricted penetration of phages through the protective matrix of biofilms, however, may limit their efficacy against biofilm infections. Here, engineered polymers were used to generate noncovalent phage-polymer nanoassemblies (PPNs) that penetrate bacterial biofilms and kill resident bacteria. Phage K, active against multiple strains of Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), was assembled with cationic poly(oxanorbornene) polymers into PPNs. The PPNs retained phage infectivity, while demonstrating enhanced biofilm penetration and killing relative to free phages. PPNs achieved 3-log10 bacterial reduction (∼99.9%) against MRSA biofilms in vitro. PPNs were then incorporated into Poloxamer 407 (P407) hydrogels and applied onto in vivo wound biofilms, demonstrating controlled and sustained release. Hydrogel-incorporated PPNs were effective in a murine MRSA wound biofilm model, showing a 1.5-log10 reduction in bacterial load compared to a 0.5 log reduction with phage K in P407 hydrogel. Overall, this work showcases the therapeutic potential of phage K engineered with cationic polymers for treating wound biofilm infections.
中文翻译:

用于治疗伤口生物膜感染的工程噬菌体-聚合物纳米组件
裂解噬菌体(噬菌体)的抗菌功效和特异性使其成为治疗多重耐药细菌感染的有前途的治疗方法。然而,噬菌体通过生物膜保护基质的渗透受限可能会限制它们对生物膜感染的疗效。在这里,工程聚合物被用于生成非共价噬菌体聚合物纳米组装体 (PPN),可穿透细菌生物膜并杀死常驻细菌。噬菌体 K 对多种金黄色葡萄球菌菌株(包括耐甲氧西林金黄色葡萄球菌 (MRSA))具有活性,与阳离子聚(氧冰烯)聚合物组装成 PPN。PPN 保留了噬菌体的感染性,同时相对于游离噬菌体表现出增强的生物膜渗透和杀伤能力。PPN 在体外实现了对 MRSA 生物膜的 3-log10 细菌减少 (∼99.9%)。然后将 PPN 掺入泊洛沙姆 407 (P407) 水凝胶中,并应用于体内伤口生物膜,证明控释和缓释。水凝胶掺入的 PPN 在小鼠 MRSA 伤口生物膜模型中有效,显示细菌载量减少 1.5 对数10,而 P407 水凝胶中使用噬菌体 K 减少 0.5 对数。总体而言,这项工作展示了用阳离子聚合物改造的噬菌体 K 治疗伤口生物膜感染的治疗潜力。
更新日期:2024-09-17
中文翻译:

用于治疗伤口生物膜感染的工程噬菌体-聚合物纳米组件
裂解噬菌体(噬菌体)的抗菌功效和特异性使其成为治疗多重耐药细菌感染的有前途的治疗方法。然而,噬菌体通过生物膜保护基质的渗透受限可能会限制它们对生物膜感染的疗效。在这里,工程聚合物被用于生成非共价噬菌体聚合物纳米组装体 (PPN),可穿透细菌生物膜并杀死常驻细菌。噬菌体 K 对多种金黄色葡萄球菌菌株(包括耐甲氧西林金黄色葡萄球菌 (MRSA))具有活性,与阳离子聚(氧冰烯)聚合物组装成 PPN。PPN 保留了噬菌体的感染性,同时相对于游离噬菌体表现出增强的生物膜渗透和杀伤能力。PPN 在体外实现了对 MRSA 生物膜的 3-log10 细菌减少 (∼99.9%)。然后将 PPN 掺入泊洛沙姆 407 (P407) 水凝胶中,并应用于体内伤口生物膜,证明控释和缓释。水凝胶掺入的 PPN 在小鼠 MRSA 伤口生物膜模型中有效,显示细菌载量减少 1.5 对数10,而 P407 水凝胶中使用噬菌体 K 减少 0.5 对数。总体而言,这项工作展示了用阳离子聚合物改造的噬菌体 K 治疗伤口生物膜感染的治疗潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号