当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Hydrogenation of Quinoline Enabled by Cu0-Cu+ Dual Sites Coupled with Efficient Biomass Valorization in Aqueous Solution
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-17 , DOI: 10.1002/adfm.202414120 Yaoling Pan 1 , Zhenyu Bao 1 , Chen Wang 1 , Zhengyu Wang 1 , Penghui Xu 1 , Xinwen Bai 1 , Xiaowei Shi 1 , Huajun Zheng 1 , Hong‐En Wang 2 , Lingxia Zheng 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-09-17 , DOI: 10.1002/adfm.202414120 Yaoling Pan 1 , Zhenyu Bao 1 , Chen Wang 1 , Zhengyu Wang 1 , Penghui Xu 1 , Xinwen Bai 1 , Xiaowei Shi 1 , Huajun Zheng 1 , Hong‐En Wang 2 , Lingxia Zheng 1
Affiliation
|
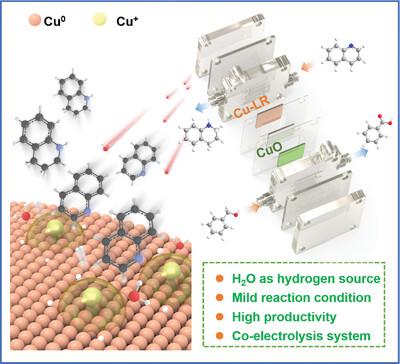
The hydrogenation of nitrogen-containing heterocyclic precursors in aqueous medium is challenging, especially at ambient temperature and pressure. Electrochemical hydrogenation (ECH) of quinoline to 1,2,3,4-tetrahydroquinoline (THQ) at mild conditions using water as the hydrogen source is demonstrated with splendid activity on a Cu-based nonprecicous catalyst. The yield of THQ is up to ≈100% with ≈100% selectivity at −1.275 V vs Hg/HgO. The real active sites and key intermediates are deciphered, where the Lewis acid-base sites on the heterointerface of Cu/Cu2O are beneficial to the quinoline adsorption in a dual-site coordination configuration and water dissociation to afford H*. The presence of Cu0 plays a vital role in inhibiting the binding of H*, which ensures good Faradaic efficiency. In addition, a novel co-production system by coupling benzyl alcohol oxidation at the anode is established, achieving dual benefits in both energy utilization efficiency and economic benefits.
中文翻译:

Cu0-Cu+ 双位点耦合水溶液中生物质增值实现喹啉的电化学加氢
含氮杂环前驱体在水性介质中的氢化具有挑战性,尤其是在环境温度和压力下。在温和条件下,使用水作为氢源将喹啉电化学加氢 (ECH) 转化为 1,2,3,4-四氢喹啉 (THQ),证明了在 Cu 基非比希催化剂上具有出色的活性。THQ 的产率高达 ≈100%,在 -1.275 V 与 Hg/HgO 相比具有 ≈100% 的选择性。破译了真正的活性位点和关键中间体,其中 Cu/Cu2O 异质界面上的 Lewis 酸碱位点有利于喹啉吸附,以双位配位构型和水解离得到 H*。Cu0 的存在在抑制 H* 的结合中起着至关重要的作用,从而确保了良好的法拉第效率。此外,建立了一种新型的联产系统,通过在阳极耦合苯甲醇氧化,实现了能源利用效率和经济效益的双重效益。
更新日期:2024-09-17
中文翻译:

Cu0-Cu+ 双位点耦合水溶液中生物质增值实现喹啉的电化学加氢
含氮杂环前驱体在水性介质中的氢化具有挑战性,尤其是在环境温度和压力下。在温和条件下,使用水作为氢源将喹啉电化学加氢 (ECH) 转化为 1,2,3,4-四氢喹啉 (THQ),证明了在 Cu 基非比希催化剂上具有出色的活性。THQ 的产率高达 ≈100%,在 -1.275 V 与 Hg/HgO 相比具有 ≈100% 的选择性。破译了真正的活性位点和关键中间体,其中 Cu/Cu2O 异质界面上的 Lewis 酸碱位点有利于喹啉吸附,以双位配位构型和水解离得到 H*。Cu0 的存在在抑制 H* 的结合中起着至关重要的作用,从而确保了良好的法拉第效率。此外,建立了一种新型的联产系统,通过在阳极耦合苯甲醇氧化,实现了能源利用效率和经济效益的双重效益。

































 京公网安备 11010802027423号
京公网安备 11010802027423号