当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical versus Non-Radical Reactivity in ortho- and para-Quinonedimethides and Implications for Cycloaddition Mechanisms
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c03001 Zhipeng Pei, Kieran P. E. Connor, Nicholas L. Magann, Michael G. Gardiner, Michelle L. Coote, Michael S. Sherburn
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c03001 Zhipeng Pei, Kieran P. E. Connor, Nicholas L. Magann, Michael G. Gardiner, Michelle L. Coote, Michael S. Sherburn
|
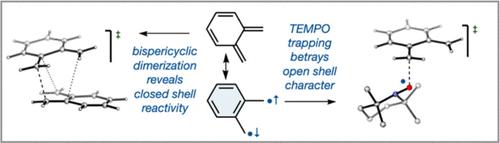
The latent singlet diradical character of the parent ortho-quinonedimethide (o-QDM), as revealed by valence bond calculations, is demonstrated experimentally by trapping with the kinetically stable free radical TEMPO at room temperature. In the absence of TEMPO, the main pathway for decomposition at ambient temperature is not (as previously proposed in the literature) a radical reaction but instead a concerted Diels–Alder dimerization, which through ωB97X-D/aug-cc-pVTZ/SMD//M06-2X-D3/6-31+G(d,p)/SMD calculations is shown to proceed through an ambimodal bispericyclic transition state. The predominantly non-radical reactivity of o-QDM at room temperature differs from that of its isomeric para-quinonedimethide (p-QDM) congener, which self-reacts exclusively through radical pathways. These findings suggest the potential for tunable concerted/stepwise cycloadditions.
中文翻译:

邻醌和对醌二甲基化物的自由基反应性与非自由基反应性以及对环加成机制的影响
通过在室温下用动力学稳定的自由基 TEMPO 捕获,通过价键计算揭示了母体邻醌二甲基化物 ( o -QDM) 的潜在单线态双自由基特征。在没有 TEMPO 的情况下,环境温度下分解的主要途径不是(如文献中先前提出的)自由基反应,而是协调一致的 Diels-Alder 二聚反应,通过 ωB97X-D/aug-cc-pVTZ/SMD/ /M06-2X-D3/6-31+G(d,p)/SMD 计算显示通过双峰双周环过渡态进行。 o -QDM 在室温下的主要非自由基反应性与其异构对醌二甲基化物 ( p -QDM) 同系物不同,后者仅通过自由基途径进行自反应。这些发现表明可调协同/逐步环加成的潜力。
更新日期:2024-09-16
中文翻译:

邻醌和对醌二甲基化物的自由基反应性与非自由基反应性以及对环加成机制的影响
通过在室温下用动力学稳定的自由基 TEMPO 捕获,通过价键计算揭示了母体邻醌二甲基化物 ( o -QDM) 的潜在单线态双自由基特征。在没有 TEMPO 的情况下,环境温度下分解的主要途径不是(如文献中先前提出的)自由基反应,而是协调一致的 Diels-Alder 二聚反应,通过 ωB97X-D/aug-cc-pVTZ/SMD/ /M06-2X-D3/6-31+G(d,p)/SMD 计算显示通过双峰双周环过渡态进行。 o -QDM 在室温下的主要非自由基反应性与其异构对醌二甲基化物 ( p -QDM) 同系物不同,后者仅通过自由基途径进行自反应。这些发现表明可调协同/逐步环加成的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号