当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent and Stereospecific Synthesis of Highly Substituted Azepines
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c03053 Yi Tian, Lei Liu, Tu Zeng, Xingcan Zhang, Baosheng Li
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c03053 Yi Tian, Lei Liu, Tu Zeng, Xingcan Zhang, Baosheng Li
|
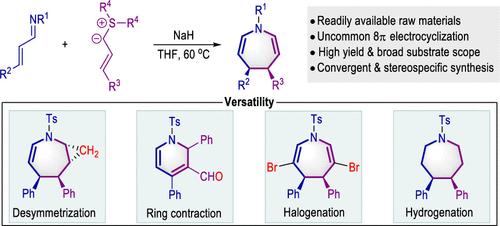
This study reported a convergent pattern to stereospecifically synthesize 4,5-dihydrogen azepine from simple and readily available starting materials, addressing synthetic and stereoselective issues. Several synthetically important transformations, such as Simmon–Smith cyclopropanation, halogenation, and hydrogenation, demonstrated the utilities of this strategy. Particularly, the final azepine products could effectively contract into highly substituted pyridine derivatives through an intramolecular oxidation rearrangement.
中文翻译:

高度取代氮杂卓的聚合和立体定向合成
这项研究报道了一种从简单易得的起始材料立体定向合成 4,5-二氢氮杂的收敛模式,解决了合成和立体选择性问题。几种重要的综合转化,例如西蒙-史密斯环丙烷化、卤化和氢化,证明了该策略的实用性。特别是,最终的氮杂卓产物可以通过分子内氧化重排有效地收缩成高度取代的吡啶衍生物。
更新日期:2024-09-16
中文翻译:

高度取代氮杂卓的聚合和立体定向合成
这项研究报道了一种从简单易得的起始材料立体定向合成 4,5-二氢氮杂的收敛模式,解决了合成和立体选择性问题。几种重要的综合转化,例如西蒙-史密斯环丙烷化、卤化和氢化,证明了该策略的实用性。特别是,最终的氮杂卓产物可以通过分子内氧化重排有效地收缩成高度取代的吡啶衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号