当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Bioorthogonal Precision Tool for Human N-Acetylglucosaminyltransferase V
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c05955 Yu Liu 1, 2 , Ganka Bineva-Todd 2 , Richard W Meek 3, 4 , Laura Mazo 5 , Beatriz Piniello 5 , Olga Moroz 3 , Sean A Burnap 6, 7 , Nadima Begum 1 , André Ohara 8 , Chloe Roustan 9 , Sara Tomita 1 , Svend Kjaer 9 , Karen Polizzi 8 , Weston B Struwe 6, 7 , Carme Rovira 5, 10 , Gideon J Davies 3 , Benjamin Schumann 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c05955 Yu Liu 1, 2 , Ganka Bineva-Todd 2 , Richard W Meek 3, 4 , Laura Mazo 5 , Beatriz Piniello 5 , Olga Moroz 3 , Sean A Burnap 6, 7 , Nadima Begum 1 , André Ohara 8 , Chloe Roustan 9 , Sara Tomita 1 , Svend Kjaer 9 , Karen Polizzi 8 , Weston B Struwe 6, 7 , Carme Rovira 5, 10 , Gideon J Davies 3 , Benjamin Schumann 1, 2
Affiliation
|
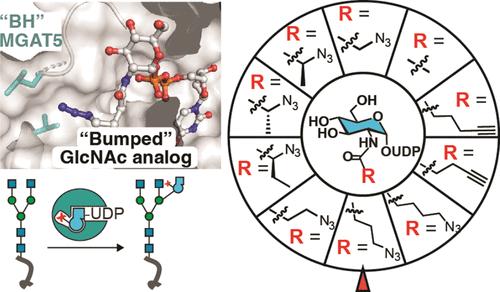
Correct elaboration of N-linked glycans in the secretory pathway of human cells is essential in physiology. Early N-glycan biosynthesis follows an assembly line principle before undergoing crucial elaboration points that feature the sequential incorporation of the sugar N-acetylglucosamine (GlcNAc). The activity of GlcNAc transferase V (MGAT5) primes the biosynthesis of an N-glycan antenna that is heavily upregulated in cancer. Still, the functional relevance and substrate choice of MGAT5 are ill-defined. Here, we employ protein engineering to develop a bioorthogonal substrate analog for the activity of MGAT5. Chemoenzymatic synthesis is used to produce a collection of nucleotide-sugar analogs with bulky, bioorthogonal acylamide side chains. We find that WT-MGAT5 displays considerable activity toward such substrate analogues. Protein engineering yields an MGAT5 variant that loses activity against the native nucleotide sugar and increases activity toward a 4-azidobutyramide-containing substrate analogue. By such restriction of substrate specificity, we show that the orthogonal enzyme–substrate pair is suitable to bioorthogonally tag glycoproteins. Through X-ray crystallography and molecular dynamics simulations, we establish the structural basis of MGAT5 engineering, informing the design rules for bioorthogonal precision chemical tools.
中文翻译:

人 N-乙酰氨基葡萄糖转移酶 V 的生物正交精密工具
正确阐述人体细胞分泌途径中的 N 连接聚糖在生理学中至关重要。早期的 N-聚糖生物合成遵循装配线原理,然后经过关键的精加工点,这些精加工点的特点是糖N-乙酰氨基葡萄糖 (GlcNAc) 的顺序掺入。 GlcNAc 转移酶 V (MGAT5) 的活性启动了 N-聚糖天线的生物合成,该天线在癌症中严重上调。尽管如此,MGAT5 的功能相关性和底物选择仍不明确。在这里,我们采用蛋白质工程来开发 MGAT5 活性的生物正交底物类似物。化学酶合成用于生产一系列具有庞大的生物正交酰胺侧链的核苷酸糖类似物。我们发现 WT-MGAT5 对此类底物类似物表现出相当大的活性。蛋白质工程产生了一种 MGAT5 变体,该变体失去了针对天然核苷酸糖的活性,并增加了针对含有 4-叠氮基丁酰胺的底物类似物的活性。通过底物特异性的这种限制,我们表明正交酶-底物对适合生物正交标记糖蛋白。通过X射线晶体学和分子动力学模拟,我们建立了MGAT5工程的结构基础,为生物正交精密化学工具的设计规则提供了信息。
更新日期:2024-09-17
中文翻译:

人 N-乙酰氨基葡萄糖转移酶 V 的生物正交精密工具
正确阐述人体细胞分泌途径中的 N 连接聚糖在生理学中至关重要。早期的 N-聚糖生物合成遵循装配线原理,然后经过关键的精加工点,这些精加工点的特点是糖N-乙酰氨基葡萄糖 (GlcNAc) 的顺序掺入。 GlcNAc 转移酶 V (MGAT5) 的活性启动了 N-聚糖天线的生物合成,该天线在癌症中严重上调。尽管如此,MGAT5 的功能相关性和底物选择仍不明确。在这里,我们采用蛋白质工程来开发 MGAT5 活性的生物正交底物类似物。化学酶合成用于生产一系列具有庞大的生物正交酰胺侧链的核苷酸糖类似物。我们发现 WT-MGAT5 对此类底物类似物表现出相当大的活性。蛋白质工程产生了一种 MGAT5 变体,该变体失去了针对天然核苷酸糖的活性,并增加了针对含有 4-叠氮基丁酰胺的底物类似物的活性。通过底物特异性的这种限制,我们表明正交酶-底物对适合生物正交标记糖蛋白。通过X射线晶体学和分子动力学模拟,我们建立了MGAT5工程的结构基础,为生物正交精密化学工具的设计规则提供了信息。































 京公网安备 11010802027423号
京公网安备 11010802027423号