当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Chemical and Structural Complexity of Electrochemical Ion Exchange in Layered Oxide Materials
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c08089 Linqin Mu 1, 2 , Dong Hou 1, 3 , Emily E Foley 4 , Minyi Dai 5 , Jin Zhang 6 , Zhisen Jiang 6 , Muhammad Mominur Rahman 1 , Yanbao Fu 7 , Lu Ma 8 , Enyuan Hu 9 , Sami Sainio 6 , Dennis Nordlund 6 , Jue Liu 10 , Jia-Mian Hu 5 , Yijin Liu 6 , Raphaële J Clément 4 , Feng Lin 1, 11
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-17 , DOI: 10.1021/jacs.4c08089 Linqin Mu 1, 2 , Dong Hou 1, 3 , Emily E Foley 4 , Minyi Dai 5 , Jin Zhang 6 , Zhisen Jiang 6 , Muhammad Mominur Rahman 1 , Yanbao Fu 7 , Lu Ma 8 , Enyuan Hu 9 , Sami Sainio 6 , Dennis Nordlund 6 , Jue Liu 10 , Jia-Mian Hu 5 , Yijin Liu 6 , Raphaële J Clément 4 , Feng Lin 1, 11
Affiliation
|
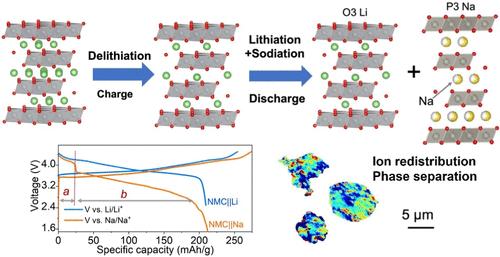
Soft chemistry techniques, such as ion exchange, hold great potential for the development of battery electrode materials that cannot be stabilized via conventional equilibrium synthesis methods. Nevertheless, the intricate mechanisms governing ion exchange remain elusive. Herein, we investigate the evolution of the long-range and local structure, as well as the ion (de)intercalation mechanism during electrochemical Li-to-Na ion exchange initiated from an O3-type lithium-layered oxide cathode. The in situ-formed mixed-cation electrolyte leads to competitive intercalation of Li and Na ions. While Li ion intercalation predominates at the beginning of initial discharge, Na ion cointercalation into a different layer results in ion redistribution and phase separation, with the emergence of a P3–Na phase alongside an O3–Li phase. Further, this study spatially resolves the heterogeneous nature of electrochemical ion exchange reactions within individual particles and provides insights into the correlations between local Ni redox processes and phase separation. Overall, electrochemical ion exchange leads to a mixed-phase cathode and alters its reaction kinetics. Those findings have important implications for the development of new metastable materials for renewable energy devices and ion separation applications.
中文翻译:

揭示层状氧化物材料中电化学离子交换的化学和结构复杂性
离子交换等软化学技术对于开发无法通过传统平衡合成方法稳定的电池电极材料具有巨大潜力。然而,控制离子交换的复杂机制仍然难以捉摸。在此,我们研究了长程和局部结构的演变,以及从 O3 型锂层状氧化物阴极引发的电化学 Li-to-Na 离子交换过程中的离子(脱)插层机制。原位形成的混合阳离子电解质导致 Li 和 Na 离子的竞争性插层。虽然锂离子嵌入在初始放电开始时占主导地位,但 Na 离子共嵌入到不同的层会导致离子重新分布和相分离,并出现 P3-Na 相和 O3-Li 相。此外,本研究在空间上解决了单个颗粒内电化学离子交换反应的异质性,并提供了对局部 Ni 氧化还原过程与相分离之间相关性的见解。总体而言,电化学离子交换导致混合相阴极并改变其反应动力学。这些发现对开发用于可再生能源设备和离子分离应用的新型亚稳态材料具有重要意义。
更新日期:2024-09-17
中文翻译:

揭示层状氧化物材料中电化学离子交换的化学和结构复杂性
离子交换等软化学技术对于开发无法通过传统平衡合成方法稳定的电池电极材料具有巨大潜力。然而,控制离子交换的复杂机制仍然难以捉摸。在此,我们研究了长程和局部结构的演变,以及从 O3 型锂层状氧化物阴极引发的电化学 Li-to-Na 离子交换过程中的离子(脱)插层机制。原位形成的混合阳离子电解质导致 Li 和 Na 离子的竞争性插层。虽然锂离子嵌入在初始放电开始时占主导地位,但 Na 离子共嵌入到不同的层会导致离子重新分布和相分离,并出现 P3-Na 相和 O3-Li 相。此外,本研究在空间上解决了单个颗粒内电化学离子交换反应的异质性,并提供了对局部 Ni 氧化还原过程与相分离之间相关性的见解。总体而言,电化学离子交换导致混合相阴极并改变其反应动力学。这些发现对开发用于可再生能源设备和离子分离应用的新型亚稳态材料具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号