当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of orthogonally protected thioamide dipeptides for use in solid-phase peptide synthesis
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-11-02 18:02:16 Kim Manzor, Fintan Kelleher
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-11-02 18:02:16 Kim Manzor, Fintan Kelleher
|
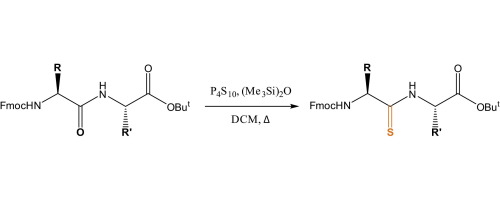
Orthogonally protected thioamide-containing dipeptides were efficiently and cleanly prepared from the precursor dipeptides using Curphey’s method (P4S10, hexamethyldisiloxane (HMDO), reflux, DCM) in 67–96% isolated yield. This was in contrast to the use of Lawesson’s or Berzelius’ reagents where significant issues with reaction non-completion, decomposition and purification were observed. Subsequent clean removal of the dipeptides’ t-butyl ester protecting groups gave thioamide dipeptide acids which were suitable for use in solid-phase peptide synthesis (SPPS).
中文翻译:

用于固相肽合成的正交保护的硫酰胺二肽的合成
使用Curphey方法(P 4 S 10,六甲基二硅氧烷(HMDO),回流,DCM)从前体二肽中高效,清洁地制备了受正交保护的含硫酰胺的二肽,分离产率为67-96%。这与使用Lawesson试剂或Berzelius试剂相反,后者在反应不完全,分解和纯化方面存在重大问题。随后干净地除去二肽的叔丁酯保护基,得到适合用于固相肽合成(SPPS)的硫代酰胺二肽酸。
更新日期:2016-11-03
中文翻译:

用于固相肽合成的正交保护的硫酰胺二肽的合成
使用Curphey方法(P 4 S 10,六甲基二硅氧烷(HMDO),回流,DCM)从前体二肽中高效,清洁地制备了受正交保护的含硫酰胺的二肽,分离产率为67-96%。这与使用Lawesson试剂或Berzelius试剂相反,后者在反应不完全,分解和纯化方面存在重大问题。随后干净地除去二肽的叔丁酯保护基,得到适合用于固相肽合成(SPPS)的硫代酰胺二肽酸。















































 京公网安备 11010802027423号
京公网安备 11010802027423号