当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Asymmetric Migratory Nozaki–Hiyama–Kishi Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c07910 Jian Chen, Lifu Wu, Zhiyong Song, Yi Wang, Zhenkun Li, You Wang, Shaolin Zhu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c07910 Jian Chen, Lifu Wu, Zhiyong Song, Yi Wang, Zhenkun Li, You Wang, Shaolin Zhu
|
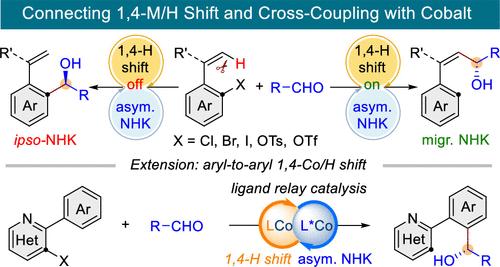
Selective functionalization of ubiquitous C–H bonds based on 1,n-metal migration provides an attractive and sustainable route to access complex molecules from readily available precursors. Herein, we report a Co-catalyzed asymmetric reductive migratory Nozaki–Hiyama–Kishi (NHK) coupling between two readily available electrophiles, aryl (pseudo)halides and aldehydes, via an unprecedented through-space aryl-to-alkenyl 1,4-cobalt/hydride shift. The judicious choice of ligands is crucial for selectivity, leading to either ipso- or migratory NHK products with exquisite control of regio-, E/Z-, and enantioselectivity. Enabled by a ligand relay catalytic strategy, this platform has been further extended to aryl-to-aryl asymmetric migratory NHK coupling. These high-value NHK adducts, including α-chiral allylic alcohols and benzyl alcohols, are readily convertible to a variety of useful synthons.
中文翻译:

钴催化的不对称迁移野崎-桧山-岸耦合
基于 1, n金属迁移的普遍存在的 C-H 键的选择性功能化提供了一种有吸引力且可持续的途径,可以从容易获得的前体中获取复杂的分子。在此,我们报道了两种容易获得的亲电子试剂芳基(拟)卤化物和醛之间的共催化不对称还原迁移Nozaki-Hiyama-Kishi(NHK)偶联,通过前所未有的穿越空间芳基到烯基1,4-钴/氢化物位移。配体的明智选择对于选择性至关重要,从而使 NHK 产品能够精确控制区域选择性、 E / Z选择性和对映选择性。通过配体中继催化策略,该平台已进一步扩展到芳基到芳基不对称迁移 NHK 偶联。这些高价值的 NHK 加合物,包括 α-手性烯丙醇和苯甲醇,很容易转化为各种有用的合成子。
更新日期:2024-09-16
中文翻译:

钴催化的不对称迁移野崎-桧山-岸耦合
基于 1, n金属迁移的普遍存在的 C-H 键的选择性功能化提供了一种有吸引力且可持续的途径,可以从容易获得的前体中获取复杂的分子。在此,我们报道了两种容易获得的亲电子试剂芳基(拟)卤化物和醛之间的共催化不对称还原迁移Nozaki-Hiyama-Kishi(NHK)偶联,通过前所未有的穿越空间芳基到烯基1,4-钴/氢化物位移。配体的明智选择对于选择性至关重要,从而使 NHK 产品能够精确控制区域选择性、 E / Z选择性和对映选择性。通过配体中继催化策略,该平台已进一步扩展到芳基到芳基不对称迁移 NHK 偶联。这些高价值的 NHK 加合物,包括 α-手性烯丙醇和苯甲醇,很容易转化为各种有用的合成子。































 京公网安备 11010802027423号
京公网安备 11010802027423号