当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH Affects the Spontaneous Formation of H2O2 at the Air–Water Interfaces
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c07356 Maria Angelaki, Jill d’Erceville, D. James Donaldson, Christian George
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c07356 Maria Angelaki, Jill d’Erceville, D. James Donaldson, Christian George
|
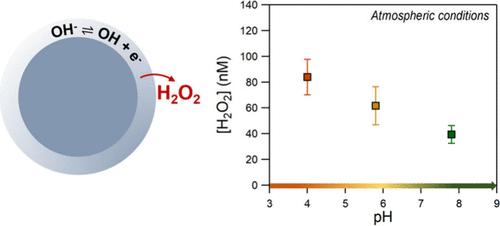
Recent studies have shown that the air–water interface of aqueous microdroplets is a source of OH radicals and hydrogen peroxide in the atmosphere. Several parameters such as droplet size, salt, and organic content have been suggested to play key roles in the formation of these oxidants. In this study, we focus on the effect of acidity on the spontaneous interfacial hydrogen peroxide formation of salt-containing droplets. Na2SO4, NaCl, and NaBr bulk solutions, at the range of pH 4 to 9.5, were nebulized, using ultra high-purity N2/O2 (80%/20%), and H2O2 was measured in the collected droplets. All of the experiments were performed in T = 292 ± 1 K and humidity levels of 90 ± 2%. For Na2SO4 and NaCl, the H2O2 concentration was increased by ∼40% under alkaline conditions, suggesting that OH– enriched environments promote its production. When CO2 was added in the ultrapure air, H2O2 was observed to be lower at higher pH. This suggests that dissolved CO2 can initiate reactions with OH radicals and electrons, impacting the interfacial H2O2 production. H2O2 formation in NaBr droplets did not display any dependence on the pH or the bath gas, showing that secondary reactions occur at the interface in the presence of Br–, which acts as an efficient interfacial source of electrons.
中文翻译:

pH 值影响 H2O2 在空气-水界面的自发形成
最近的研究表明,水性微滴的空气-水界面是大气中 OH 自由基和过氧化氢的来源。液滴大小、盐和有机物含量等几个参数被认为在这些氧化剂的形成中起关键作用。在这项研究中,我们专注于酸度对含盐液滴自发界面过氧化氢形成的影响。使用超高纯度 N2/O2 (80%/20%) 雾化 pH 值为 4 至 9.5 的 Na2SO4、NaCl 和 NaBr 散装溶液,并测量收集的液滴中的 H2O2。所有实验均在 T = 292 ± 1 K 和 90 ± 2% 的湿度水平下进行。对于 Na2SO4 和 NaCl,H2O2 浓度在碱性条件下增加了 ∼40%,表明富含 OH 的环境促进了其产生。当在超纯空气中加入 CO2 时,观察到 H2O2 在较高 pH 值下较低。这表明溶解的 CO2 可以引发与 OH 自由基和电子的反应,从而影响界面 H2O2 的产生。NaBr 液滴中 H2O2 的形成对 pH 值或浴液气体没有任何依赖性,表明在 Br 存在下,界面处发生二次反应–Br–充当有效的电子界面源。
更新日期:2024-09-16
中文翻译:

pH 值影响 H2O2 在空气-水界面的自发形成
最近的研究表明,水性微滴的空气-水界面是大气中 OH 自由基和过氧化氢的来源。液滴大小、盐和有机物含量等几个参数被认为在这些氧化剂的形成中起关键作用。在这项研究中,我们专注于酸度对含盐液滴自发界面过氧化氢形成的影响。使用超高纯度 N2/O2 (80%/20%) 雾化 pH 值为 4 至 9.5 的 Na2SO4、NaCl 和 NaBr 散装溶液,并测量收集的液滴中的 H2O2。所有实验均在 T = 292 ± 1 K 和 90 ± 2% 的湿度水平下进行。对于 Na2SO4 和 NaCl,H2O2 浓度在碱性条件下增加了 ∼40%,表明富含 OH 的环境促进了其产生。当在超纯空气中加入 CO2 时,观察到 H2O2 在较高 pH 值下较低。这表明溶解的 CO2 可以引发与 OH 自由基和电子的反应,从而影响界面 H2O2 的产生。NaBr 液滴中 H2O2 的形成对 pH 值或浴液气体没有任何依赖性,表明在 Br 存在下,界面处发生二次反应–Br–充当有效的电子界面源。































 京公网安备 11010802027423号
京公网安备 11010802027423号