当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Theoretical Assessment of Covalency in a Pu(III) Borohydride Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c09888 Joshua C. Zgrabik, Daniel J. Lussier, Rina Bhowmick, Ngan Nguyen, Peter A. Zacher III, Tatyana Elkin, Andrew J. Gaunt, George S. Goff, Harris E. Mason, Jesse Murillo, Brian L. Scott, Bess Vlaisavljevich, Scott R. Daly
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c09888 Joshua C. Zgrabik, Daniel J. Lussier, Rina Bhowmick, Ngan Nguyen, Peter A. Zacher III, Tatyana Elkin, Andrew J. Gaunt, George S. Goff, Harris E. Mason, Jesse Murillo, Brian L. Scott, Bess Vlaisavljevich, Scott R. Daly
|
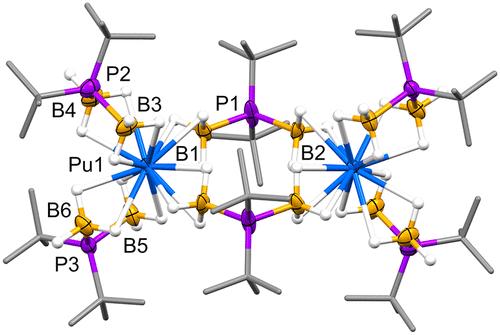
Despite the discovery of actinide borohydride complexes over 80 years ago, no plutonium borohydride complexes have been structurally validated using single-crystal X-ray diffraction (XRD). Here we describe Pu2(H3BPtBu2BH3)6, the first example of a Pu(III) borohydride complex authenticated by XRD and NMR spectroscopy. Theoretical calculations (DFT, EDA, and QTAIM) and experimental comparisons of metal–boron distances suggest that metal–borohydride covalency in M2(H3BPtBu2BH3)6 complexes generally decreases in the order M = U(III) > Pu(III) > Ln(III).
中文翻译:

Pu(III) 硼氢化物配合物共价的结构和理论评估
尽管 80 多年前就发现了锕系硼氢化物配合物,但尚未使用单晶 X 射线衍射 (XRD) 对硼氢化钚配合物进行结构验证。在这里,我们描述了 Pu2(H3BPtBu2BH3)6,这是通过 XRD 和 NMR 波谱鉴定的 Pu(III) 硼氢化物络合物的第一个例子。理论计算(DFT、EDA 和 QTAIM)和金属-硼距离的实验比较表明,M2(H3BPtBu2BH3)6 配合物中的金属-硼氢化物共价通常以 M = U(III) > Pu(III) > Ln(III) 的顺序降低。
更新日期:2024-09-16
中文翻译:

Pu(III) 硼氢化物配合物共价的结构和理论评估
尽管 80 多年前就发现了锕系硼氢化物配合物,但尚未使用单晶 X 射线衍射 (XRD) 对硼氢化钚配合物进行结构验证。在这里,我们描述了 Pu2(H3BPtBu2BH3)6,这是通过 XRD 和 NMR 波谱鉴定的 Pu(III) 硼氢化物络合物的第一个例子。理论计算(DFT、EDA 和 QTAIM)和金属-硼距离的实验比较表明,M2(H3BPtBu2BH3)6 配合物中的金属-硼氢化物共价通常以 M = U(III) > Pu(III) > Ln(III) 的顺序降低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号