当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Insecticidal Activity of Isoxazoline Derivatives Incorporating an Acylhydrazine Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.jafc.4c04005 Biaobiao Jiang, Di Feng, Jun Shi, Wei Wu, Yawen Dong, Hai Ren
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.jafc.4c04005 Biaobiao Jiang, Di Feng, Jun Shi, Wei Wu, Yawen Dong, Hai Ren
|
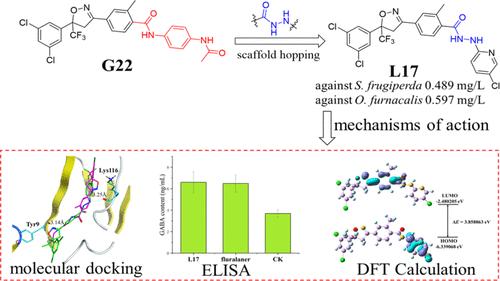
The long-term use of agricultural insecticides has led to the development of resistant strains. In this context, the isoxazoline structure has become an active area of pesticide research owing to its wide insecticidal spectrum, nontoxicity to mammals, and lack of cross-resistance with known insecticides. In the present study, based on the discovery of compound G22 in our previous work, a series of novel isoxazoline compounds containing acylhydrazine were designed and synthesized using a scaffold hopping strategy. The insecticidal activities of the target compounds were assessed, and compound L17 (LC50 = 0.489 mg/L) showed insecticidal activity against Spodoptera frugiperda superior to those of the commercial insecticides indoxacarb (LC50 = 3.14 mg/L) and fluralaner (LC50 = 0.659 mg/L). Theoretical calculations indicated that the introduction of acylhydrazine plays an important role in the biological activity of the target compounds. Furthermore, the enzyme-linked immunosorbent assay and molecular docking results indicated that L17 may act on the GABA receptor of the target insect. These results indicated that L17 is a potential candidate compound for controlling S. frugiperda populations in agriculture.
中文翻译:

含有酰肼部分的异恶唑啉衍生物的设计、合成和杀虫活性
农用杀虫剂的长期使用导致了抗药性菌株的产生。在此背景下,异恶唑啉结构因其杀虫谱广、对哺乳动物无毒且与已知杀虫剂不存在交叉耐药性而成为农药研究的活跃领域。本研究基于前期工作中化合物G22的发现,采用支架跳跃策略设计合成了一系列含有酰肼的新型异恶唑啉化合物。对目标化合物的杀虫活性进行了评估,化合物L17 (LC 50 = 0.489 mg/L)对草地贪夜蛾的杀虫活性优于市售杀虫剂茚虫威(LC 50 = 3.14 mg/L)和氟乐兰(LC 50) = 0.659 毫克/升)。理论计算表明酰肼的引入对目标化合物的生物活性起着重要作用。此外,酶联免疫吸附试验和分子对接结果表明, L17可能作用于目标昆虫的GABA受体。这些结果表明, L17是控制农业中草地贪夜蛾种群的潜在候选化合物。
更新日期:2024-09-16
中文翻译:

含有酰肼部分的异恶唑啉衍生物的设计、合成和杀虫活性
农用杀虫剂的长期使用导致了抗药性菌株的产生。在此背景下,异恶唑啉结构因其杀虫谱广、对哺乳动物无毒且与已知杀虫剂不存在交叉耐药性而成为农药研究的活跃领域。本研究基于前期工作中化合物G22的发现,采用支架跳跃策略设计合成了一系列含有酰肼的新型异恶唑啉化合物。对目标化合物的杀虫活性进行了评估,化合物L17 (LC 50 = 0.489 mg/L)对草地贪夜蛾的杀虫活性优于市售杀虫剂茚虫威(LC 50 = 3.14 mg/L)和氟乐兰(LC 50) = 0.659 毫克/升)。理论计算表明酰肼的引入对目标化合物的生物活性起着重要作用。此外,酶联免疫吸附试验和分子对接结果表明, L17可能作用于目标昆虫的GABA受体。这些结果表明, L17是控制农业中草地贪夜蛾种群的潜在候选化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号