当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Regioselective C(sp2)–H Bond Chalcogenation of Pyrazolo[1,5-a]pyrimidines via Radical Cross-Coupling at Room Temperature
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.joc.4c00856 Abhinay S. Chillal, Rajesh T. Bhawale, Siddharth Sharma, Umesh A. Kshirsagar
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.joc.4c00856 Abhinay S. Chillal, Rajesh T. Bhawale, Siddharth Sharma, Umesh A. Kshirsagar
|
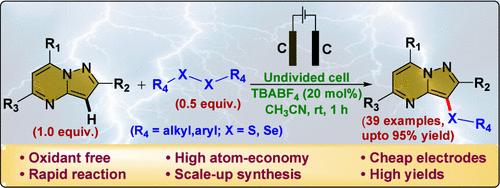
Herein, we disclose an electrochemical approach for the C(sp2)–H chalcogenation of pyrazolo[1,5-a]pyrimidines. This technique offers an oxidant and catalyst-free protocol for achieving regioselective chalcogenation of pyrazolo[1,5-a]pyrimidines. The procedure uses only 0.5 equiv. of diaryl chalcogenides which underscores the atom economy of the protocol. Key attributes of this methodology include mild reaction conditions, short reaction time, utilization of cheap electrode materials, and eco-friendly reaction conditions. Cyclic voltammetry studies and radical quenching experiments revealed a radical cross-coupling pathway for the reaction mechanism.
中文翻译:

在室温下通过自由基交叉偶联对吡唑并[1,5-a]嘧啶进行电化学区域选择性 C(sp2)–H 键硫化反应
在此,我们公开了吡唑并[1,5-a]嘧啶的 C(sp2)-H 硫化学发生方法。该技术提供了一种无氧化剂和无催化剂的方案,用于实现吡唑并[1,5-a]嘧啶的区域选择性硫化。该程序仅使用 0.5 当量的二芳基硫属化物,这强调了该方案的原子经济性。该方法的主要特点包括温和的反应条件、短反应时间、使用廉价的电极材料和环保的反应条件。循环伏安法研究和自由基猝灭实验揭示了反应机理的自由基交叉耦合途径。
更新日期:2024-09-16
中文翻译:

在室温下通过自由基交叉偶联对吡唑并[1,5-a]嘧啶进行电化学区域选择性 C(sp2)–H 键硫化反应
在此,我们公开了吡唑并[1,5-a]嘧啶的 C(sp2)-H 硫化学发生方法。该技术提供了一种无氧化剂和无催化剂的方案,用于实现吡唑并[1,5-a]嘧啶的区域选择性硫化。该程序仅使用 0.5 当量的二芳基硫属化物,这强调了该方案的原子经济性。该方法的主要特点包括温和的反应条件、短反应时间、使用廉价的电极材料和环保的反应条件。循环伏安法研究和自由基猝灭实验揭示了反应机理的自由基交叉耦合途径。













































 京公网安备 11010802027423号
京公网安备 11010802027423号