当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Regioselective Redox-Neutral 1,3-Oxypyridylation of Aryl Cyclopropanes
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c02918 Dong-Jie Li, Xia-Ling Liu, You-Zhi Liao, Yi Zhao, Fei Pan
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c02918 Dong-Jie Li, Xia-Ling Liu, You-Zhi Liao, Yi Zhao, Fei Pan
|
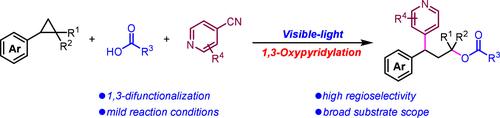
Pyridines and cyclopropanes are important structural units in chemistry. Herein, we introduce a photoredox-catalyzed approach for the ring opening and 1,3-oxypyridylation of aryl cyclopropanes using 4-cyanopyridines and carboxylic acids. This sequential process involves single-electron oxidation of the aryl cyclopropane, leading to nucleophilic ring opening and radical pyridylation at the benzylic position. The redox-neutral reaction exhibits high regioselectivity under mild reaction conditions, offering a broad substrate scope and wide applicability.
中文翻译:

芳基环丙烷的光催化区域选择性氧化还原中性 1,3-氧化吡啶化
吡啶和环丙烷是化学中重要的结构单元。在此,我们介绍了一种使用 4-氰基吡啶和羧酸进行芳基环丙烷开环和 1,3-氧基吡啶化的光氧化还原催化方法。该连续过程涉及芳基环丙烷的单电子氧化,导致亲核开环和在苄基位置上的自由基吡啶化。氧化还原中性反应在温和的反应条件下表现出较高的区域选择性,提供了广泛的底物范围和广泛的适用性。
更新日期:2024-09-16
中文翻译:

芳基环丙烷的光催化区域选择性氧化还原中性 1,3-氧化吡啶化
吡啶和环丙烷是化学中重要的结构单元。在此,我们介绍了一种使用 4-氰基吡啶和羧酸进行芳基环丙烷开环和 1,3-氧基吡啶化的光氧化还原催化方法。该连续过程涉及芳基环丙烷的单电子氧化,导致亲核开环和在苄基位置上的自由基吡啶化。氧化还原中性反应在温和的反应条件下表现出较高的区域选择性,提供了广泛的底物范围和广泛的适用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号