当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C═N Bond Ozonolysis and β-Scission: A Breakthrough Approach to the Synthesis of ω-Functionalized Compounds from Carbonyl Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c02999 Dmitri I. Fomenkov, Roman A. Budekhin, Peter S. Radulov, Alexander I. Fomenkov, Liang-Nian He, Ivan A. Yaremenko, Alexander O. Terent’ev
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.orglett.4c02999 Dmitri I. Fomenkov, Roman A. Budekhin, Peter S. Radulov, Alexander I. Fomenkov, Liang-Nian He, Ivan A. Yaremenko, Alexander O. Terent’ev
|
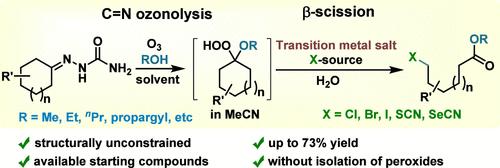
This work discloses a two-step, one-pot approach to ω-functionalized esters via cleavage of the alicyclic fragment of cycloalkanone semicarbazones. This approach is based on a combination of the synthesis of various alkoxyhydroperoxides via cycloalkanone semicarbazone ozonolysis and in situ interaction of these peroxides with transition metal salts, leading to cleavage of the aliphatic cycle and subsequent ω-functionalized ester formation. A broad series of ω-halogen or pseudohalogen esters have been successfully synthesized in yields ranging from 23 to 73% per starting semicarbazone. A major advantage of the approach is the ability to use different cycloalkanone semicarbazones, including those with large cycles and substituents in them. The possibility of carrying out ozonolysis in the presence of various alcohols makes it possible to obtain the corresponding esters of ω-substituted carboxylic acids.
中文翻译:

C=N键臭氧分解和β-断裂:从羰基衍生物合成ω-官能化化合物的突破性方法
这项工作公开了一种通过环烷酮缩氨基脲的脂环片段裂解来制备 ω-官能化酯的两步一锅法。该方法基于通过环烷酮缩氨基脲臭氧分解合成各种烷氧基氢过氧化物以及这些过氧化物与过渡金属盐的原位相互作用的组合,导致脂肪族环的裂解和随后的ω-官能化酯的形成。已成功合成一系列 ω-卤素或拟卤素酯,每个起始缩氨基脲的收率范围为 23% 至 73%。该方法的一个主要优点是能够使用不同的环烷酮缩氨基脲,包括其中具有大环和取代基的环烷酮缩氨基脲。在各种醇的存在下进行臭氧分解的可能性使得可以获得相应的ω-取代的羧酸的酯。
更新日期:2024-09-16
中文翻译:

C=N键臭氧分解和β-断裂:从羰基衍生物合成ω-官能化化合物的突破性方法
这项工作公开了一种通过环烷酮缩氨基脲的脂环片段裂解来制备 ω-官能化酯的两步一锅法。该方法基于通过环烷酮缩氨基脲臭氧分解合成各种烷氧基氢过氧化物以及这些过氧化物与过渡金属盐的原位相互作用的组合,导致脂肪族环的裂解和随后的ω-官能化酯的形成。已成功合成一系列 ω-卤素或拟卤素酯,每个起始缩氨基脲的收率范围为 23% 至 73%。该方法的一个主要优点是能够使用不同的环烷酮缩氨基脲,包括其中具有大环和取代基的环烷酮缩氨基脲。在各种醇的存在下进行臭氧分解的可能性使得可以获得相应的ω-取代的羧酸的酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号