当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Amination of Aryl Chlorides under Mild Reaction Conditions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c10237 Han-Jun Ai, Seoung-Tae Kim, Cecilia Liu, Stephen L. Buchwald
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c10237 Han-Jun Ai, Seoung-Tae Kim, Cecilia Liu, Stephen L. Buchwald
|
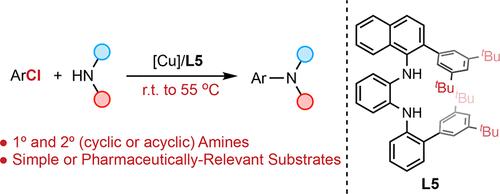
We report a mild method for the copper-catalyzed amination of aryl chlorides. Key to the success of the method was the use of highly sterically encumbered N1,N2-diaryl diamine ligands which resist catalyst deactivation, allowing reactions to proceed at significantly lower temperatures and with a broader scope than current protocols. A sequence of highly chemoselective C–N and C–O cross-coupling reactions were demonstrated, and mechanistic studies indicate that oxidative addition of the Cu catalyst to the aryl chlorides is rate-limiting. We anticipate that the design principles disclosed herein will help motivate further advances in Cu-catalyzed transformations of aryl chlorides.
中文翻译:

在温和反应条件下铜催化芳基氯化物胺化反应
我们报道了一种用于铜催化芳基氯化物胺化反应的温和方法。该方法成功的关键是使用高度空间位阻的 N1,N 2-二芳基二胺配体,这些配体可抵抗催化剂失活,允许反应在明显较低的温度下进行,并且范围比当前方案更广。证明了一系列高化学选择性的 C-N 和 C-O 交叉偶联反应,机理研究表明 Cu 催化剂与芳基氯化物的氧化加成是限速的。我们预计本文披露的设计原则将有助于推动 Cu 催化芳基氯化物转化的进一步进展。
更新日期:2024-09-16
中文翻译:

在温和反应条件下铜催化芳基氯化物胺化反应
我们报道了一种用于铜催化芳基氯化物胺化反应的温和方法。该方法成功的关键是使用高度空间位阻的 N1,N 2-二芳基二胺配体,这些配体可抵抗催化剂失活,允许反应在明显较低的温度下进行,并且范围比当前方案更广。证明了一系列高化学选择性的 C-N 和 C-O 交叉偶联反应,机理研究表明 Cu 催化剂与芳基氯化物的氧化加成是限速的。我们预计本文披露的设计原则将有助于推动 Cu 催化芳基氯化物转化的进一步进展。































 京公网安备 11010802027423号
京公网安备 11010802027423号