当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lighting Up Nonemissive Azobenzene Derivatives by Pressure
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-15 , DOI: 10.1021/jacs.4c09784 Shuhe Hu, Xiu Yin, Shuang Liu, Yuye Yan, Jiahui Mu, Haichao Liu, Qiuyan Cen, Min Wu, Long Lv, Ran Liu, Haiyan Li, Mingguang Yao, Ruiyang Zhao, Dong Yao, Bo Zou, Guangtian Zou, Yuguang Ma
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-15 , DOI: 10.1021/jacs.4c09784 Shuhe Hu, Xiu Yin, Shuang Liu, Yuye Yan, Jiahui Mu, Haichao Liu, Qiuyan Cen, Min Wu, Long Lv, Ran Liu, Haiyan Li, Mingguang Yao, Ruiyang Zhao, Dong Yao, Bo Zou, Guangtian Zou, Yuguang Ma
|
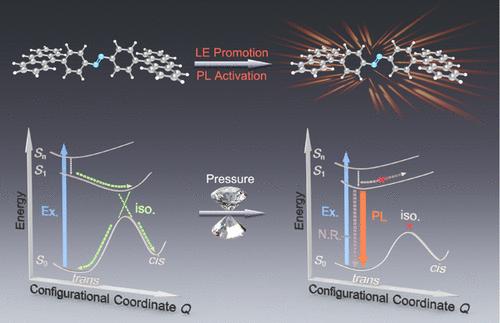
Pressure-induced emission (PIE) is a compelling phenomenon that can activate luminescence within nonemissive materials. However, PIE in nonemissive organic materials has never been achieved. Herein, we present the first observation of PIE in an organic system, specifically within nonemissive azobenzene derivatives. The emission of 1,2-bis(4-(anthracen-9-yl)phenyl)diazene was activated at 0.52 GPa, primarily driven by local excitation promotion induced by molecular conformational changes. Complete photoisomerization suppression of the molecule was observed at 1.5 GPa, concurrently accelerating the emission enhancement to 3.53 GPa. Differing from the key role of isomerization inhibition in conventional perception, our findings demonstrate that the excited-state constituent is the decisive factor for emission activation, providing a potentially universal approach for high-efficiency azobenzene emission. Additionally, PIE was replicated in the analogue 1,2-bis(4-(9H-carbazol-9-yl)phenyl)diazene, confirming the general applicability of our findings. This work marks a significant breakthrough within the PIE paradigm and paves the novel high-pressure route for crystalline-state photoisomerization investigation.
中文翻译:

通过压力点亮非自发光偶氮苯衍生物
压力诱导发射 (PIE) 是一种引人注目的现象,可以在非发射材料中激活发光。然而,非发射有机材料中的 PIE 从未实现过。在本文中,我们首次在有机系统中观察到 PIE,特别是在非发射偶氮苯衍生物中。1,2-双(4-(蒽-9-基)苯基)二氮烯的发射在 0.52 GPa 时被激活,主要由分子构象变化诱导的局部激发促进驱动。在 1.5 GPa 时观察到分子的完全光异构化抑制,同时加速发射增强至 3.53 GPa。与传统感知中异构化抑制的关键作用不同,我们的研究结果表明,激发态成分是发射激活的决定性因素,为高效偶氮苯排放提供了一种潜在的通用方法。此外,PIE 在类似物 1,2-双(4-(9H-碳唑-9-基)苯基)二氮烯中复制,证实了我们研究结果的普遍适用性。这项工作标志着 PIE 范式的重大突破,并为结晶态光异构化研究铺平了新的高压路线。
更新日期:2024-09-15
中文翻译:

通过压力点亮非自发光偶氮苯衍生物
压力诱导发射 (PIE) 是一种引人注目的现象,可以在非发射材料中激活发光。然而,非发射有机材料中的 PIE 从未实现过。在本文中,我们首次在有机系统中观察到 PIE,特别是在非发射偶氮苯衍生物中。1,2-双(4-(蒽-9-基)苯基)二氮烯的发射在 0.52 GPa 时被激活,主要由分子构象变化诱导的局部激发促进驱动。在 1.5 GPa 时观察到分子的完全光异构化抑制,同时加速发射增强至 3.53 GPa。与传统感知中异构化抑制的关键作用不同,我们的研究结果表明,激发态成分是发射激活的决定性因素,为高效偶氮苯排放提供了一种潜在的通用方法。此外,PIE 在类似物 1,2-双(4-(9H-碳唑-9-基)苯基)二氮烯中复制,证实了我们研究结果的普遍适用性。这项工作标志着 PIE 范式的重大突破,并为结晶态光异构化研究铺平了新的高压路线。































 京公网安备 11010802027423号
京公网安备 11010802027423号