当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Modular Approach for Accessing 3D Heterocycles via 1,2-Dicyanation of Planar N-Heteroarenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-09-16 , DOI: 10.1002/anie.202412979 Sukumar Pradhan, Sudip Maiti, Suparna Dutta, C. Adam Russell, Sameer Tyagi, Debabrata Maiti
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-09-16 , DOI: 10.1002/anie.202412979 Sukumar Pradhan, Sudip Maiti, Suparna Dutta, C. Adam Russell, Sameer Tyagi, Debabrata Maiti
|
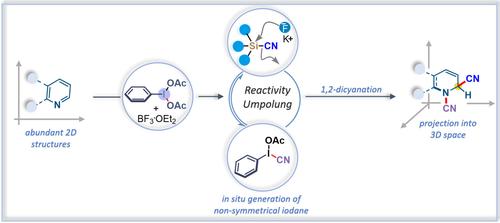
A dearomative functionalization approach of abundant N-heteroarenes has been developed to harvest valued 3D N-heterocyclic skeletons. This robust method is enabled by the in situ generation of highly reactive non-symmetric iodane from bench-stable hypervalent iodane, PhI(OAc)2, and BF3 ⋅ OEt2 A wide range of N-heteroarenes were transformed into their corresponding 3D analog via the installation of a highly versatile cyano group as a new vector.
中文翻译:

一种通过平面 N-杂芳烃的 1,2-二氰化获得 3D 杂环的模块化方法
已经开发了一种丰富的 N-杂芳烃的去浪漫功能化方法来收获有价值的 3D N-杂环骨架。这种稳健的方法是通过从台式稳定的高价碘 PhI(OAc)2 和 BF3 ⋅ OEt2 原位生成高反应性非对称碘来实现的。通过安装高度通用的氰基作为新载体,将各种 N-杂芳烃转化为相应的 3D 类似物。
更新日期:2024-09-16
中文翻译:

一种通过平面 N-杂芳烃的 1,2-二氰化获得 3D 杂环的模块化方法
已经开发了一种丰富的 N-杂芳烃的去浪漫功能化方法来收获有价值的 3D N-杂环骨架。这种稳健的方法是通过从台式稳定的高价碘 PhI(OAc)2 和 BF3 ⋅ OEt2 原位生成高反应性非对称碘来实现的。通过安装高度通用的氰基作为新载体,将各种 N-杂芳烃转化为相应的 3D 类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号