Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Monitoring the Nucleation and Growth of Nanoscale CaCO3 at the Oil–Water Interface
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-16 , DOI: 10.1021/acsnano.4c02490 Yaguang Zhu, Ying Wang, Zhenwei Gao, Prashant Gupta, Srikanth Singamaneni, Xiaobing Zuo, Young-Shin Jun
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-16 , DOI: 10.1021/acsnano.4c02490 Yaguang Zhu, Ying Wang, Zhenwei Gao, Prashant Gupta, Srikanth Singamaneni, Xiaobing Zuo, Young-Shin Jun
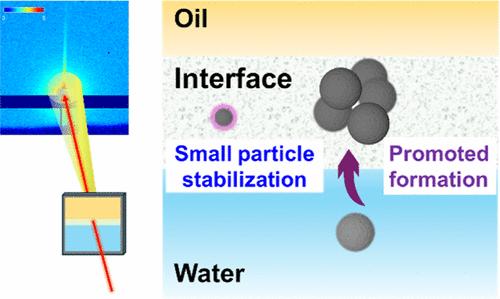
|
Interfaces can actively control the nucleation kinetics, orientations, and polymorphs of calcium carbonate (CaCO3). Prior studies have revealed that CaCO3 formation can be affected by the interplay between chemical functional moieties on solid–liquid or air–liquid interfaces as well as CaCO3’s precursors and facets. Yet little is known about the roles of a liquid–liquid interface, specifically an oil–liquid interface, in directing CaCO3 mineralization which are common in natural and engineered systems. Here, by using in situ X-ray scattering techniques to locate a meniscus formed between water and a representative oil, isooctane, we successfully monitored CaCO3 formation at the pliable isooctane–water interface and systematically investigated the pivotal roles of the interface in the formation of CaCO3 (i.e., particle size, its spatial distribution with respect to the interface, and its mineral phase). Different from bulk solution, ∼5 nm CaCO3 nanoparticles form at the isooctane–water interface. They stably exist for a long time (36 h), which can result from interface-stabilized dehydrated prenucleation clusters of CaCO3. There is a clear tendency for enhanced amounts and faster crystallization of CaCO3 at locations closer to isooctane, which is attributed to a higher pH and an easier dehydration environment created by the interface and oil. Our study provides insights into CaCO3 nucleation at an oil–water interface, which can deepen our understanding of pliable interfaces interacting with CaCO3 and benefit mineral scaling control during energy-related subsurface operation.
中文翻译:

原位监测油-水界面处纳米级 CaCO3 的成核和生长
界面可以主动控制碳酸钙 (CaCO3) 的成核动力学、取向和多晶型物。先前的研究表明,CaCO3 的形成会受到固-液或气-液界面上化学功能部分以及 CaCO3 的前体和刻面之间相互作用的影响。然而,人们对液-液界面,特别是油-液界面,在引导 CaCO3 矿化中的作用知之甚少,这在自然和工程系统中很常见。在这里,通过使用原位 X 射线散射技术定位在水和代表性油异辛烷之间形成的弯月面,我们成功监测了 CaCO3 在柔韧的异辛烷-水界面处的形成,并系统地研究了界面在 CaCO3 形成中的关键作用(即颗粒大小、其相对于界面的空间分布、 及其矿物相)。与本体溶液不同,∼5 nm CaCO3 纳米颗粒在异辛烷-水界面形成。它们稳定存在很长时间 (36 h),这可能是由 CaCO3 的界面稳定脱水前成核簇引起的。在更接近异辛烷的位置,CaCO3 的结晶量增加和结晶速度明显增加,这归因于更高的 pH 值以及界面和油产生的更容易脱水的环境。我们的研究提供了对油-水界面处 CaCO3 成核的见解,这可以加深我们对与 CaCO3 相互作用的柔韧界面的理解,并有利于在与能源相关的地下作业期间控制矿物结垢。
更新日期:2024-09-16
中文翻译:

原位监测油-水界面处纳米级 CaCO3 的成核和生长
界面可以主动控制碳酸钙 (CaCO3) 的成核动力学、取向和多晶型物。先前的研究表明,CaCO3 的形成会受到固-液或气-液界面上化学功能部分以及 CaCO3 的前体和刻面之间相互作用的影响。然而,人们对液-液界面,特别是油-液界面,在引导 CaCO3 矿化中的作用知之甚少,这在自然和工程系统中很常见。在这里,通过使用原位 X 射线散射技术定位在水和代表性油异辛烷之间形成的弯月面,我们成功监测了 CaCO3 在柔韧的异辛烷-水界面处的形成,并系统地研究了界面在 CaCO3 形成中的关键作用(即颗粒大小、其相对于界面的空间分布、 及其矿物相)。与本体溶液不同,∼5 nm CaCO3 纳米颗粒在异辛烷-水界面形成。它们稳定存在很长时间 (36 h),这可能是由 CaCO3 的界面稳定脱水前成核簇引起的。在更接近异辛烷的位置,CaCO3 的结晶量增加和结晶速度明显增加,这归因于更高的 pH 值以及界面和油产生的更容易脱水的环境。我们的研究提供了对油-水界面处 CaCO3 成核的见解,这可以加深我们对与 CaCO3 相互作用的柔韧界面的理解,并有利于在与能源相关的地下作业期间控制矿物结垢。































 京公网安备 11010802027423号
京公网安备 11010802027423号