Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbohydrate-Lectin Interactions Reprogram Dendritic Cells to Promote Type 1 Anti-Tumor Immunity
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-16 , DOI: 10.1021/acsnano.4c07360 Valerie Lensch, Adele Gabba, Robert Hincapie, Sachin H. Bhagchandani, Ankit Basak, Mohammad Murshid Alam, Jeffery Noble, Darrell J. Irvine, Alex K. Shalek, Jeremiah A. Johnson, M. G. Finn, Laura L. Kiessling
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-16 , DOI: 10.1021/acsnano.4c07360 Valerie Lensch, Adele Gabba, Robert Hincapie, Sachin H. Bhagchandani, Ankit Basak, Mohammad Murshid Alam, Jeffery Noble, Darrell J. Irvine, Alex K. Shalek, Jeremiah A. Johnson, M. G. Finn, Laura L. Kiessling
|
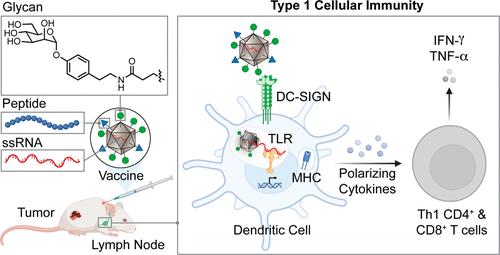
Cancer vaccine development is inhibited by a lack of strategies for directing dendritic cell (DC) induction of effective tumor-specific cellular immunity. Pathogen engagement of DC lectins and toll-like receptors (TLRs) is thought to shape immunity by directing T cell function. Controlling downstream responses, however, remains a major challenge. A critical goal in advancing vaccine development involves the identification of receptors that drive type 1 cellular immunity. The immune system monitors cells for aberrant glycosylation (a sign of a foreign entity), but potent activation occurs when a second signal, such as single-stranded RNA or lipopolysaccharide, is present to activate TLR signaling. To exploit dual signaling, we engineered a glycan-costumed virus-like particle (VLP) vaccine that displays a DC-SIGN-selective aryl mannose ligand and encapsulates TLR7 agonists. These VLPs deliver programmable peptide antigens to induce robust DC activation and type 1 cellular immunity. In contrast, VLPs lacking this critical DC-SIGN ligand promoted DC-mediated humoral immunity, offering limited tumor control. Vaccination with glycan-costumed VLPs generated tumor antigen-specific Th1 CD4+ and CD8+ T cells that infiltrated solid tumors, significantly inhibiting tumor growth in a murine melanoma model. The tailored VLPs also afforded protection against the reintroduction of tumor cells. Thus, DC lectin-driven immune reprogramming, combined with the modular programmability of VLP platforms, provides a promising framework for directing cellular immunity to advance cancer immunotherapies and vaccines.
中文翻译:

碳水化合物-凝集素相互作用重编程树突状细胞以促进 1 型抗肿瘤免疫
由于缺乏指导树突状细胞 (DC) 诱导有效肿瘤特异性细胞免疫的策略,癌症疫苗的开发受到抑制。DC 凝集素和 toll 样受体 (TLR) 的病原体参与被认为通过指导 T 细胞功能来塑造免疫力。然而,控制下游反应仍然是一个重大挑战。推进疫苗开发的一个关键目标涉及鉴定驱动 1 型细胞免疫的受体。免疫系统监测细胞的异常糖基化(外来实体的迹象),但当存在第二个信号(如单链 RNA 或脂多糖)以激活 TLR 信号时,就会发生有效的激活。为了利用双重信号传导,我们设计了一种聚糖修饰的病毒样颗粒 (VLP) 疫苗,该疫苗显示 DC-SIGN 选择性芳基甘露糖配体并封装 TLR7 激动剂。这些 VLP 提供可编程的肽抗原,以诱导强大的 DC 激活和 1 型细胞免疫。相比之下,缺乏这种关键 DC-SIGN 配体的 VLP 促进了 DC 介导的体液免疫,从而提供了有限的肿瘤控制。用聚糖装化的 VLP 接种疫苗会产生肿瘤抗原特异性 Th1 CD4 + 和 CD8 + T 细胞,这些细胞浸润实体瘤,显着抑制小鼠黑色素瘤模型中的肿瘤生长。定制的 VLP 还提供了防止肿瘤细胞重新引入的保护。因此,DC 凝集素驱动的免疫重编程与 VLP 平台的模块化可编程性相结合,为指导细胞免疫以推进癌症免疫疗法和疫苗提供了一个有前途的框架。
更新日期:2024-09-16
中文翻译:

碳水化合物-凝集素相互作用重编程树突状细胞以促进 1 型抗肿瘤免疫
由于缺乏指导树突状细胞 (DC) 诱导有效肿瘤特异性细胞免疫的策略,癌症疫苗的开发受到抑制。DC 凝集素和 toll 样受体 (TLR) 的病原体参与被认为通过指导 T 细胞功能来塑造免疫力。然而,控制下游反应仍然是一个重大挑战。推进疫苗开发的一个关键目标涉及鉴定驱动 1 型细胞免疫的受体。免疫系统监测细胞的异常糖基化(外来实体的迹象),但当存在第二个信号(如单链 RNA 或脂多糖)以激活 TLR 信号时,就会发生有效的激活。为了利用双重信号传导,我们设计了一种聚糖修饰的病毒样颗粒 (VLP) 疫苗,该疫苗显示 DC-SIGN 选择性芳基甘露糖配体并封装 TLR7 激动剂。这些 VLP 提供可编程的肽抗原,以诱导强大的 DC 激活和 1 型细胞免疫。相比之下,缺乏这种关键 DC-SIGN 配体的 VLP 促进了 DC 介导的体液免疫,从而提供了有限的肿瘤控制。用聚糖装化的 VLP 接种疫苗会产生肿瘤抗原特异性 Th1 CD4 + 和 CD8 + T 细胞,这些细胞浸润实体瘤,显着抑制小鼠黑色素瘤模型中的肿瘤生长。定制的 VLP 还提供了防止肿瘤细胞重新引入的保护。因此,DC 凝集素驱动的免疫重编程与 VLP 平台的模块化可编程性相结合,为指导细胞免疫以推进癌症免疫疗法和疫苗提供了一个有前途的框架。

































 京公网安备 11010802027423号
京公网安备 11010802027423号