当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries: Mechanisms, Challenges, and Strategies
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.accounts.4c00484 Kaiyue Zhu, Weishen Yang
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/acs.accounts.4c00484 Kaiyue Zhu, Weishen Yang
|
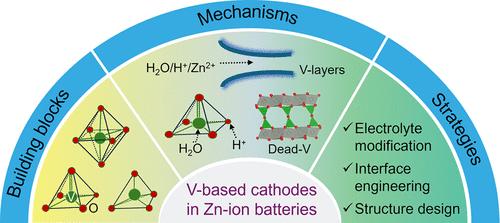
Zinc-ion batteries (ZIBs) are highly promising for large-scale energy storage because of their safety, high energy/power density, low cost, and eco-friendliness. Vanadium-based compounds are attractive cathodes because of their versatile structures and multielectron redox processes (+5 to +3), leading to high capacity. Layered structures or 3-dimensional open tunnel frameworks allow easy movement of zinc-ions without breaking the structure apart, offering superior rate-performance. However, challenges such as dissolution and phase transformation hinder the long-term stability of vanadium-based cathodes in ZIBs. Although significant research has been dedicated to understanding the mechanisms and developing high-performance vanadium-based cathodes, uncertainties still exist regarding the critical mechanisms of energy storage and dissolution, the actual active phase and the specific optimization strategy. For example, it is unclear whether materials such as α-V2O5, VO2, and V2O3 serve as the active phase or undergo phase transformations during cycling. Additionally, the root cause of V-dissolution and the role of byproducts such as Zn3(OH)2V2O7·2H2O in ZIBs are debated.
中文翻译:

用于水性锌离子电池的钒基阴极:机制、挑战和策略
锌离子电池 (ZIB) 因其安全性、高能量/功率密度、低成本和环保性而极具前景,可用于大规模储能。钒基化合物因其多功能结构和多电子氧化还原工艺(+5 至 +3)而成为有吸引力的阴极,从而实现高容量。分层结构或 3 维开放式隧道框架允许锌离子轻松移动,而不会将结构分开,从而提供卓越的倍率性能。然而,溶解和相变等挑战阻碍了钒基阴极在 ZIB 中的长期稳定性。尽管人们已经进行了大量研究来了解钒基阴极的机制和开发高性能钒基阴极,但关于能量存储和溶解的关键机制、实际的活性相和具体的优化策略仍然存在不确定性。例如,目前尚不清楚 α-V2O5、VO2 和 V2O3 等材料在循环过程中是用作活性相还是发生相变。此外,还讨论了 V 溶解的根本原因以及 Zn3(OH)2V2O7·2H2O 等副产物在 ZIB 中的作用。
更新日期:2024-09-16
中文翻译:

用于水性锌离子电池的钒基阴极:机制、挑战和策略
锌离子电池 (ZIB) 因其安全性、高能量/功率密度、低成本和环保性而极具前景,可用于大规模储能。钒基化合物因其多功能结构和多电子氧化还原工艺(+5 至 +3)而成为有吸引力的阴极,从而实现高容量。分层结构或 3 维开放式隧道框架允许锌离子轻松移动,而不会将结构分开,从而提供卓越的倍率性能。然而,溶解和相变等挑战阻碍了钒基阴极在 ZIB 中的长期稳定性。尽管人们已经进行了大量研究来了解钒基阴极的机制和开发高性能钒基阴极,但关于能量存储和溶解的关键机制、实际的活性相和具体的优化策略仍然存在不确定性。例如,目前尚不清楚 α-V2O5、VO2 和 V2O3 等材料在循环过程中是用作活性相还是发生相变。此外,还讨论了 V 溶解的根本原因以及 Zn3(OH)2V2O7·2H2O 等副产物在 ZIB 中的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号