当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silyl Radicals as Single-Electron Reductants: α-Aminoalkyl Radical Formation via a Photocatalytic Oxidatively Initiated Radical Chain Process
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c08230 Harry C. Waller, Matthew J. Gaunt
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-16 , DOI: 10.1021/jacs.4c08230 Harry C. Waller, Matthew J. Gaunt
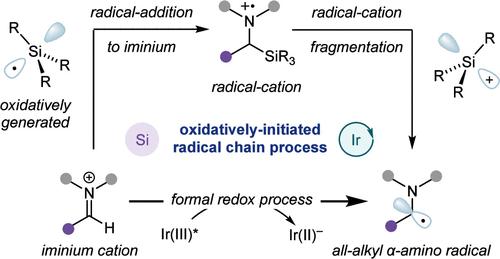
|
The α-amino-radical constitutes a versatile reactive intermediate that has been used to great effect in the synthesis of complex amine-containing products. Here, we report the development of a multicomponent photocatalytic platform enabling access to all-alkyl α-amino-radicals, exploiting the oxidative formation of silyl-radicals from commercially available tris(trimethylsilyl)silane. A key design element of the new process involves the role of silyl-radicals in generating α-amino-radicals from iminium ions as part of an oxidatively initiated photocatalytic radical chain process. This distinct activation mode is showcased by engaging the ensuing radicals in cross-radical coupling with persistent arene radical anions, enabling the arylation of in situ-generated all-alkyl iminium ions to furnish alkyl-substituted benzylamines.
中文翻译:

甲硅烷基自由基作为单电子还原剂:通过光催化氧化引发的自由基链过程形成 α-氨基烷基自由基
α-氨基自由基构成了一种多功能反应中间体,在复杂的含胺产品的合成中发挥了巨大作用。在此,我们报告了一种多组分光催化平台的开发,该平台能够利用市售三(三甲基甲硅烷基)硅烷氧化形成甲硅烷基自由基,从而获得全烷基α-氨基自由基。新工艺的一个关键设计元素涉及甲硅烷基自由基在从亚胺离子生成α-氨基自由基中的作用,作为氧化引发的光催化自由基链过程的一部分。这种独特的活化模式是通过使随后的自由基与持久的芳烃自由基阴离子进行交叉自由基偶联来展示的,从而能够使原位生成的全烷基亚胺离子芳基化以提供烷基取代的苄胺。
更新日期:2024-09-16
中文翻译:

甲硅烷基自由基作为单电子还原剂:通过光催化氧化引发的自由基链过程形成 α-氨基烷基自由基
α-氨基自由基构成了一种多功能反应中间体,在复杂的含胺产品的合成中发挥了巨大作用。在此,我们报告了一种多组分光催化平台的开发,该平台能够利用市售三(三甲基甲硅烷基)硅烷氧化形成甲硅烷基自由基,从而获得全烷基α-氨基自由基。新工艺的一个关键设计元素涉及甲硅烷基自由基在从亚胺离子生成α-氨基自由基中的作用,作为氧化引发的光催化自由基链过程的一部分。这种独特的活化模式是通过使随后的自由基与持久的芳烃自由基阴离子进行交叉自由基偶联来展示的,从而能够使原位生成的全烷基亚胺离子芳基化以提供烷基取代的苄胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号