当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-support interaction in supported Pt single-atom catalyst promotes lattice oxygen activation to achieve complete oxidation of acetone at low concentrations
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-09-15 , DOI: 10.1016/j.jhazmat.2024.135839 Tian Tang , ShunZheng Zhao , YunPeng Liu , XiaoLong Tang , Long Sun , YiMing Ma , RongHui Zhu , Hong-Hong Yi
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-09-15 , DOI: 10.1016/j.jhazmat.2024.135839 Tian Tang , ShunZheng Zhao , YunPeng Liu , XiaoLong Tang , Long Sun , YiMing Ma , RongHui Zhu , Hong-Hong Yi
|
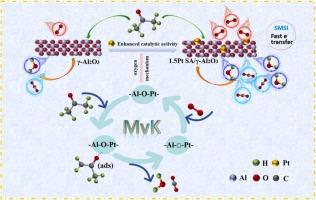
A precious metal catalyst with loaded Pt single atoms was prepared and used for the complete oxidation of C3 H6 O. Detailed results show that the T100 of the 1.5Pt SA/γ-Al2 O3 catalyst in the oxidation process of acetone is 250 °C, the TOF of Pt is 1.09 × 10−2 s−1 , and the catalyst exhibits good stability. Characterization reveals that the high dispersion of Pt single atoms and strong interaction with the carrier improve the redox properties of the catalyst, enhancing the adsorption and dissociation capability of gaseous oxygen. DFT calculations show that after the introduction of Pt, the oxygen vacancy formation energy on the catalyst surface is reduced to 1.2 eV, and PDOS calculations prove that electrons on Pt atoms can be quickly transferred to O atoms, increasing the number of electrons on the σ p * bond and promoting the escape of lattice oxygen. In addition, in situ DRIFTS and adsorption experiments indicate that the C3 H6 O oxidation process follows the Mars-van Krevelen reaction mechanism, and CH2 =C(CH3 )=O(ads) , O* (O2 - ), formate, acetate, and carbonate are considered as the main intermediate species and/or transients in the reaction process. Particularly, the activation rate of O2 and the cleavage of the -C-C- bond are the main rate-determining steps in the oxidation of C3 H6 O. This work will further enhance the study of the oxidation mechanism of oxygenated volatile organic pollutants over loaded noble metal catalysts.
中文翻译:

负载型 Pt 单原子催化剂中的金属-载体相互作用促进晶格氧活化,从而在低浓度下实现丙酮的完全氧化
制备了负载了 Pt 单原子的贵金属催化剂,用于 C3H6O 的完全氧化。详细结果表明,1.5Pt SA/γ-Al2O3催化剂在丙酮氧化过程中的T100为250 °C,Pt的TOF为1.09 × 10−2 s−1,催化剂表现出良好的稳定性。表征表明,Pt 单原子的高分散性以及与载流子的强相互作用改善了催化剂的氧化还原性能,增强了气态氧的吸附和解离能力。DFT 计算表明,引入 Pt 后,催化剂表面的氧空位形成能降低到 1.2 eV,PDOS 计算证明 Pt 原子上的电子可以迅速转移到 O 原子上,增加 σp * 键上的电子数,促进晶格氧的逸出。此外,原位 DRIFTS 和吸附实验表明,C3H6O 氧化过程遵循 Mars-van Krevelen 反应机制,CH2 =C(CH3)=O(ads)、O* (O2-)、甲酸盐、乙酸盐和碳酸盐被认为是反应过程中的主要中间物质和/或瞬变物质。特别是,O2 的活化速率和 -CC- 键的裂解是 C3H6O 氧化的主要速率决定步骤。这项工作将进一步加强对含氧挥发性有机污染物对负载贵金属催化剂的氧化机理的研究。
更新日期:2024-09-15
中文翻译:

负载型 Pt 单原子催化剂中的金属-载体相互作用促进晶格氧活化,从而在低浓度下实现丙酮的完全氧化
制备了负载了 Pt 单原子的贵金属催化剂,用于 C3H6O 的完全氧化。详细结果表明,1.5Pt SA/γ-Al2O3催化剂在丙酮氧化过程中的T100为250 °C,Pt的TOF为1.09 × 10−2 s−1,催化剂表现出良好的稳定性。表征表明,Pt 单原子的高分散性以及与载流子的强相互作用改善了催化剂的氧化还原性能,增强了气态氧的吸附和解离能力。DFT 计算表明,引入 Pt 后,催化剂表面的氧空位形成能降低到 1.2 eV,PDOS 计算证明 Pt 原子上的电子可以迅速转移到 O 原子上,增加 σp * 键上的电子数,促进晶格氧的逸出。此外,原位 DRIFTS 和吸附实验表明,C3H6O 氧化过程遵循 Mars-van Krevelen 反应机制,CH2 =C(CH3)=O(ads)、O* (O2-)、甲酸盐、乙酸盐和碳酸盐被认为是反应过程中的主要中间物质和/或瞬变物质。特别是,O2 的活化速率和 -CC- 键的裂解是 C3H6O 氧化的主要速率决定步骤。这项工作将进一步加强对含氧挥发性有机污染物对负载贵金属催化剂的氧化机理的研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号