当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional and Mechanistic Dissection of Protein Glutaminase PG3 and Its Rational Engineering for Enhanced Modification of Myofibrillar Proteins
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.jafc.4c05590 Weijun Leng 1, 2 , Ying Li 1, 3 , Li Yuan 1 , Xiuting Li 2 , Ruichang Gao 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-09-13 , DOI: 10.1021/acs.jafc.4c05590 Weijun Leng 1, 2 , Ying Li 1, 3 , Li Yuan 1 , Xiuting Li 2 , Ruichang Gao 1
Affiliation
|
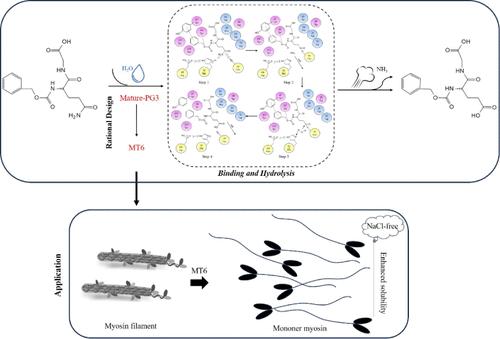
Protein glutaminases (PG; EC = 3.5.1.44) are enzymes known for enhancing protein functionality. In this study, we cloned and expressed the gene chryb3 encoding protein glutaminase PG3, exhibiting 39.4 U/mg specific activity. Mature-PG3 featured a substrate channel surrounded by aromatic and hydrophobic amino acids at positions 38–45 and 78–84, with Val81 playing a pivotal role in substrate affinity. The dynamic opening and closing motions between Gly65, Thr66, and Cys164 at the catalytic cleft greatly influence substrate binding and product release. Redesigning catalytic pocket and cocatalytic region produced combinatorial mutant MT6 showing a 2.69-fold increase in specific activity and a 2.99-fold increase at t65 °C1/2. Furthermore, MT6 boosted fish myofibrillar protein (MP) solubility without NaCl. Key residues such as Thr3, Asn54, Val81, Tyr82, Asn107, and Ser108 were vital for PG3-myosin interaction, particularly Asn54 and Asn107. This study sheds light on the catalytic mechanism of PG3 and guided its rational engineering and utilization in low-salt fish MP product production.
中文翻译:

蛋白质谷氨酰胺酶 PG3 的功能和机制解析及其增强肌原纤维蛋白修饰的合理工程
蛋白质谷氨酰胺酶(PG;EC = 3.5.1.44)是已知可增强蛋白质功能的酶。在本研究中,我们克隆并表达了编码蛋白谷氨酰胺酶PG3的基因chryb 3,表现出39.4 U/mg的比活性。 Mature-PG3 在 38-45 和 78-84 位具有被芳香族和疏水性氨基酸包围的底物通道,其中 Val81 在底物亲和力中发挥着关键作用。催化裂隙处 Gly65、Thr66 和 Cys164 之间的动态打开和关闭运动极大地影响底物结合和产物释放。重新设计催化袋和共催化区域产生了组合突变体MT6,其比活性增加了2.69倍,并且在t 65 °C 1/2时增加了2.99倍。此外,MT6 在没有 NaCl 的情况下提高了鱼肌原纤维蛋白 (MP) 的溶解度。 Thr3、Asn54、Val81、Tyr82、Asn107 和 Ser108 等关键残基对于 PG3-肌球蛋白相互作用至关重要,特别是 Asn54 和 Asn107。该研究揭示了PG3的催化机制,并指导其在低盐鱼MP产品生产中的合理设计和应用。
更新日期:2024-09-13
中文翻译:

蛋白质谷氨酰胺酶 PG3 的功能和机制解析及其增强肌原纤维蛋白修饰的合理工程
蛋白质谷氨酰胺酶(PG;EC = 3.5.1.44)是已知可增强蛋白质功能的酶。在本研究中,我们克隆并表达了编码蛋白谷氨酰胺酶PG3的基因chryb 3,表现出39.4 U/mg的比活性。 Mature-PG3 在 38-45 和 78-84 位具有被芳香族和疏水性氨基酸包围的底物通道,其中 Val81 在底物亲和力中发挥着关键作用。催化裂隙处 Gly65、Thr66 和 Cys164 之间的动态打开和关闭运动极大地影响底物结合和产物释放。重新设计催化袋和共催化区域产生了组合突变体MT6,其比活性增加了2.69倍,并且在t 65 °C 1/2时增加了2.99倍。此外,MT6 在没有 NaCl 的情况下提高了鱼肌原纤维蛋白 (MP) 的溶解度。 Thr3、Asn54、Val81、Tyr82、Asn107 和 Ser108 等关键残基对于 PG3-肌球蛋白相互作用至关重要,特别是 Asn54 和 Asn107。该研究揭示了PG3的催化机制,并指导其在低盐鱼MP产品生产中的合理设计和应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号