Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping Full Conformational Transition Dynamics of Intrinsically Disordered Proteins Using a Single-Molecule Nanocircuit
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acsnano.4c04064 Dongbao Yin 1, 2 , Ruoyao Xiong 1 , Zhiheng Yang 1, 2 , Jianfei Feng 1 , Wenzhe Liu 1 , Shiyun Li 1 , Mingyao Li 1 , Hao Ruan 1 , Jie Li 1 , Lidong Li 2 , Luhua Lai 1, 3, 4 , Xuefeng Guo 1, 5, 6
ACS Nano ( IF 15.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acsnano.4c04064 Dongbao Yin 1, 2 , Ruoyao Xiong 1 , Zhiheng Yang 1, 2 , Jianfei Feng 1 , Wenzhe Liu 1 , Shiyun Li 1 , Mingyao Li 1 , Hao Ruan 1 , Jie Li 1 , Lidong Li 2 , Luhua Lai 1, 3, 4 , Xuefeng Guo 1, 5, 6
Affiliation
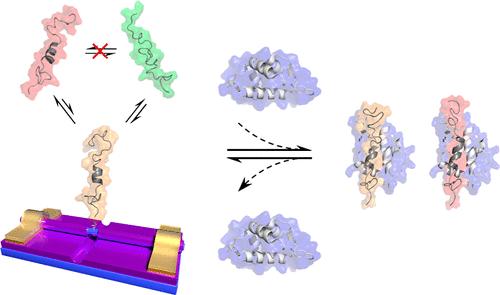
|
Intrinsically disordered proteins (IDPs) are emerging therapeutic targets for human diseases. However, probing their transient conformations remains challenging because of conformational heterogeneity. To address this problem, we developed a biosensor using a point-functionalized silicon nanowire (SiNW) that allows for real-time sampling of single-molecule dynamics. A single IDP, N-terminal transactivation domain of tumor suppressor protein p53 (p53TAD1), was covalently conjugated to the SiNW through chemical engineering, and its conformational transition dynamics was characterized as current fluctuations. Furthermore, when a globular protein ligand in solution bound to the targeted p53TAD1, protein–protein interactions could be unambiguously distinguished from large-amplitude current signals. These proof-of-concept experiments enable semiquantitative, realistic characterization of the structural properties of IDPs and constitute the basis for developing a valuable tool for protein profiling and drug discovery in the future.
中文翻译:

使用单分子纳米电路绘制本质无序蛋白质的完整构象转变动力学
内在无序蛋白(IDP)是人类疾病的新兴治疗靶点。然而,由于构象异质性,探测它们的瞬时构象仍然具有挑战性。为了解决这个问题,我们开发了一种使用点功能化硅纳米线(SiNW)的生物传感器,可以对单分子动力学进行实时采样。通过化学工程将肿瘤抑制蛋白 p53 (p53 TAD1 ) 的 N 端反式激活结构域 IDP 与 SiNW 共价结合,其构象转变动力学以电流波动为特征。此外,当溶液中的球状蛋白配体与目标 p53 TAD1结合时,可以将蛋白质间相互作用与大振幅电流信号明确区分开。这些概念验证实验能够对 IDP 的结构特性进行半定量、真实的表征,并为未来开发用于蛋白质分析和药物发现的有价值的工具奠定了基础。
更新日期:2024-09-14
中文翻译:

使用单分子纳米电路绘制本质无序蛋白质的完整构象转变动力学
内在无序蛋白(IDP)是人类疾病的新兴治疗靶点。然而,由于构象异质性,探测它们的瞬时构象仍然具有挑战性。为了解决这个问题,我们开发了一种使用点功能化硅纳米线(SiNW)的生物传感器,可以对单分子动力学进行实时采样。通过化学工程将肿瘤抑制蛋白 p53 (p53 TAD1 ) 的 N 端反式激活结构域 IDP 与 SiNW 共价结合,其构象转变动力学以电流波动为特征。此外,当溶液中的球状蛋白配体与目标 p53 TAD1结合时,可以将蛋白质间相互作用与大振幅电流信号明确区分开。这些概念验证实验能够对 IDP 的结构特性进行半定量、真实的表征,并为未来开发用于蛋白质分析和药物发现的有价值的工具奠定了基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号