当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Host–Guest Interactions for Electrochemically Stable and Thermally Safe Lithium Metal Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-12 , DOI: 10.1021/acsenergylett.4c01583 Jia-Xin Guo 1 , Feng Jiang 1 , Nai-Lu Shen 1 , Wen-Bo Tang 1, 2 , Tao Wang 1 , Yuan Ma 1 , Yiren Zhong 1 , Jiarui He 1 , Zhi Zhu 1 , Faxing Wang 1 , Xin-Bing Cheng 1, 2 , Yuping Wu 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-12 , DOI: 10.1021/acsenergylett.4c01583 Jia-Xin Guo 1 , Feng Jiang 1 , Nai-Lu Shen 1 , Wen-Bo Tang 1, 2 , Tao Wang 1 , Yuan Ma 1 , Yiren Zhong 1 , Jiarui He 1 , Zhi Zhu 1 , Faxing Wang 1 , Xin-Bing Cheng 1, 2 , Yuping Wu 1
Affiliation
|
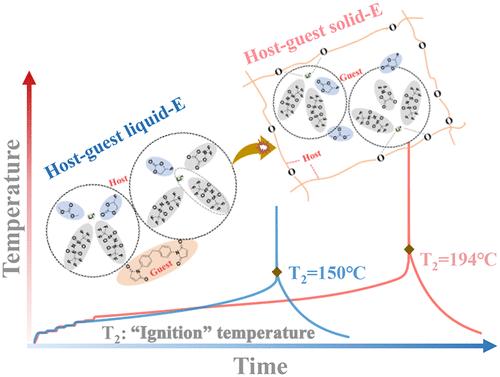
Safety concerns have been a long-standing barrier hindering widespread applications of lithium metal batteries. Herein, we introduce host–guest interactions to regulate the working models of electrolytes with a built-in safety switch. At ambient temperature, the host–guest liquid electrolyte with high-concentration-electrolyte host and polymer monomer 1,1′-(methylenedi-4,1- phenylene)bismaleimide guest renders Li||LiNi0.5Co0.2Mn0.3O2 (3 mAh cm–2) coin battery 87% capacity retention after 210 cycles due to the regulations in the solvation structure and anode interface. When facing thermal safety hazards, polymer monomers undergo molecular conformation change. It rapidly constructs a thermally stable polymer matrix with various binding sites of lithium ions to host the high-concentration electrolyte, enabling batteries to work when they cool to room temperature. Under extreme abuse conditions, thermal runaway triggering temperatures of 2.0 Ah cycled pouch batteries are increased from 150 to 194 °C. The host–guest interactions are highly effective in constructing electrochemically stable and thermally safe lithium metal batteries.
中文翻译:

电化学稳定和热安全锂金属电池的主客体相互作用
安全问题一直是阻碍锂金属电池广泛应用的长期障碍。在本文中,我们引入了主客体交互,以使用内置安全开关调节电解质的工作模型。在环境温度下,具有高浓度电解质主和聚合物单体 1,1′-(亚甲二-4,1-苯基)双马来酰亚胺的主客体液体电解质使 Li||LiNi0.5Co0.2Mn0.3O2 (3 mAh cm–2) 纽扣电池 由于溶剂化结构和阳极界面的规定,210 次循环后容量保持 87%。当面临热安全危害时,聚合物单体会发生分子构象变化。它快速构建具有各种锂离子结合位点的热稳定聚合物基质,以承载高浓度电解质,使电池能够在冷却至室温时工作。在极端滥用条件下,2.0 Ah 循环软包电池的热失控触发温度从 150 °C 提高到 194 °C。 主客体相互作用在构建电化学稳定和热安全的锂金属电池方面非常有效。
更新日期:2024-09-12
中文翻译:

电化学稳定和热安全锂金属电池的主客体相互作用
安全问题一直是阻碍锂金属电池广泛应用的长期障碍。在本文中,我们引入了主客体交互,以使用内置安全开关调节电解质的工作模型。在环境温度下,具有高浓度电解质主和聚合物单体 1,1′-(亚甲二-4,1-苯基)双马来酰亚胺的主客体液体电解质使 Li||LiNi0.5Co0.2Mn0.3O2 (3 mAh cm–2) 纽扣电池 由于溶剂化结构和阳极界面的规定,210 次循环后容量保持 87%。当面临热安全危害时,聚合物单体会发生分子构象变化。它快速构建具有各种锂离子结合位点的热稳定聚合物基质,以承载高浓度电解质,使电池能够在冷却至室温时工作。在极端滥用条件下,2.0 Ah 循环软包电池的热失控触发温度从 150 °C 提高到 194 °C。 主客体相互作用在构建电化学稳定和热安全的锂金属电池方面非常有效。































 京公网安备 11010802027423号
京公网安备 11010802027423号