当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Site Catalysts for the Oxygen Reduction Reaction: Why Iron Is Better than Platinum
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-09-14 , DOI: 10.1021/acscatal.4c02366 Alessandro Facchin 1 , Daniel Forrer 1, 2 , Mirco Zerbetto 1 , Francesco Cazzadori 1 , Andrea Vittadini 1, 2 , Christian Durante 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-09-14 , DOI: 10.1021/acscatal.4c02366 Alessandro Facchin 1 , Daniel Forrer 1, 2 , Mirco Zerbetto 1 , Francesco Cazzadori 1 , Andrea Vittadini 1, 2 , Christian Durante 1
Affiliation
|
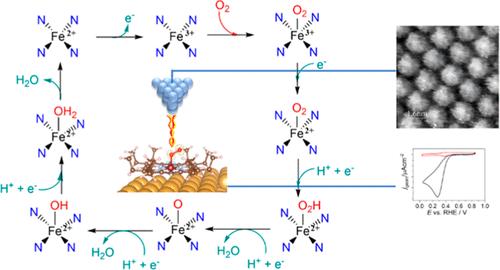
This paper aims to elucidate the origin and the different catalytic properties toward the oxygen reduction reaction in the acidic electrolyte of Fe–octaethylporphyrin (FeOEt) and Pt–octaethylporphyrin (PtOEt) supported on Au(111) electrodes. The electrocatalytic process in the two systems is monitored by using in situ scanning tunneling microscopy, allowing observation of the different redox states of the metal center and the different coordination of oxygen, which manifests as a net difference in topography. The coordination of oxygen at the two metal centers was confirmed by computational models, which observed a much stronger interaction between Fe–O2 (1.75 Å) than in Pt–O2 (3 Å). Cyclic voltammetry at still and rotating ring disc electrodes evidenced that at FeOEP, the ORR occurred according to redox-catalysis-like, Eonset (ORR) = 0.5 V vs reversible hydrogen electrode (RHE), where the variation of the metal center redox state mediates the reduction of the oxygen molecule, recovering its original oxidation state by reduction at the electrode. Conversely, PtOEP, which does not possess a redox behavior, results in worse performances, Eonset (ORR) = 0.275 V vs RHE, but certain catalysis is still observed. A tetraelectronic reduction process to H2O was observed at both metal centers, and the mechanism was fully interpreted by computational analysis.
中文翻译:

氧还原反应的单位催化剂:为什么铁优于铂
本文旨在阐明 Au(111) 电极上负载的 Fe-八乙基卟啉 (FeOEt) 和 Pt-八乙基卟啉 (PtOEt) 的酸性电解质中氧还原反应的起源和不同的催化性质。通过使用原位扫描隧道显微镜监测两个系统中的电催化过程,可以观察金属中心的不同氧化还原状态和氧的不同配位,这表现为形貌的净差异。计算模型证实了两个金属中心的氧配位,该模型观察到 Fe-O2 (1.75 Å) 之间的相互作用比 Pt-O2 (3 Å) 强得多。静止和旋转环盘电极的循环伏安法证明,在 FeOEP 下,ORR 根据氧化还原催化样 E起始 (ORR) = 0.5 V 与可逆氢电极 (RHE) 发生,其中金属中心氧化还原态的变化介导氧分子的还原,通过在电极处还原恢复其原始氧化态。相反,不具有氧化还原行为的 PtOEP 会导致性能变差,E发作 (ORR) = 0.275 V vs RHE,但仍观察到某些催化作用。在两个金属中心都观察到四电子还原过程为 H2O,并且通过计算分析完全解释了其机制。
更新日期:2024-09-14
中文翻译:

氧还原反应的单位催化剂:为什么铁优于铂
本文旨在阐明 Au(111) 电极上负载的 Fe-八乙基卟啉 (FeOEt) 和 Pt-八乙基卟啉 (PtOEt) 的酸性电解质中氧还原反应的起源和不同的催化性质。通过使用原位扫描隧道显微镜监测两个系统中的电催化过程,可以观察金属中心的不同氧化还原状态和氧的不同配位,这表现为形貌的净差异。计算模型证实了两个金属中心的氧配位,该模型观察到 Fe-O2 (1.75 Å) 之间的相互作用比 Pt-O2 (3 Å) 强得多。静止和旋转环盘电极的循环伏安法证明,在 FeOEP 下,ORR 根据氧化还原催化样 E起始 (ORR) = 0.5 V 与可逆氢电极 (RHE) 发生,其中金属中心氧化还原态的变化介导氧分子的还原,通过在电极处还原恢复其原始氧化态。相反,不具有氧化还原行为的 PtOEP 会导致性能变差,E发作 (ORR) = 0.275 V vs RHE,但仍观察到某些催化作用。在两个金属中心都观察到四电子还原过程为 H2O,并且通过计算分析完全解释了其机制。













































 京公网安备 11010802027423号
京公网安备 11010802027423号