当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of a Polypeptide Inhibitor of O-GlcNAc Transferase with Picomolar Affinity
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-14 , DOI: 10.1021/jacs.4c08656 Forrest A Hammel 1 , N Connor Payne 2, 3 , Victoria M Marando 1 , Ralph Mazitschek 2, 4, 5 , Suzanne Walker 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-09-14 , DOI: 10.1021/jacs.4c08656 Forrest A Hammel 1 , N Connor Payne 2, 3 , Victoria M Marando 1 , Ralph Mazitschek 2, 4, 5 , Suzanne Walker 1
Affiliation
|
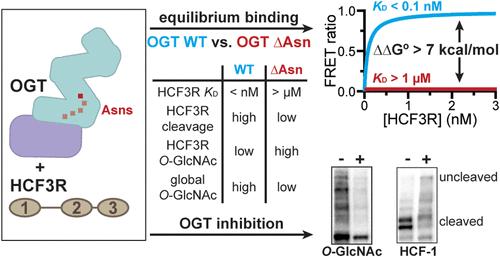
O-GlcNAc transferase (OGT) is an essential mammalian enzyme that binds thousands of different proteins, including substrates that it glycosylates and nonsubstrate interactors that regulate its biology. OGT also has one proteolytic substrate, the transcriptional coregulator host cell factor 1 (HCF-1), which it cleaves in a process initiated by glutamate side chain glycosylation at a series of central repeats. Although HCF-1 is OGT’s most prominent binding partner, its affinity for the enzyme has not been quantified. Here, we report a time-resolved Förster resonance energy transfer assay to measure ligand binding to OGT and show that an HCF-1-derived polypeptide (HCF3R) binds with picomolar affinity to the enzyme (KD ≤ 85 pM). This high affinity is driven in large part by conserved asparagines in OGT’s tetratricopeptide repeat domain, which form bidentate contacts to the HCF-1 peptide backbone; replacing any one of these asparagines with alanine reduces binding by more than 5 orders of magnitude. Because the HCF-1 polypeptide binds so tightly to OGT, we tested its ability to inhibit enzymatic function. We found that HCF3R potently inhibits OGT both in vitro and in cells and used this finding to develop a genetically encoded, inducible OGT inhibitor that can be degraded with a small molecule, allowing for reversible and tunable inhibition of OGT.
中文翻译:

鉴定具有 Picomolar 亲和力的 O-GlcNAc 转移酶多肽抑制剂
O-GlcNAc 转移酶 (OGT) 是一种必需的哺乳动物酶,可结合数千种不同的蛋白质,包括它糖基化的底物和调节其生物学的非底物相互作用物。OGT 还具有一种蛋白水解底物,即转录共调节宿主细胞因子 1 (HCF-1),它在谷氨酸侧链糖基化引发的一系列中央重复序列中裂解。尽管 HCF-1 是 OGT 最突出的结合伴侣,但它对酶的亲和力尚未被量化。在这里,我们报道了一种时间分辨的 Förster 共振能量转移测定法,用于测量配体与 OGT 的结合,并表明 HCF-1 衍生的多肽 (HCF3R) 与酶 (KD≤ 85 pM) 以皮摩尔亲和力结合。这种高亲和力在很大程度上是由 OGT 四肽重复结构域中的保守天冬酰胺驱动的,这些天冬酰胺与 HCF-1 肽骨架形成双齿接触;用丙氨酸替换这些天冬酰胺中的任何一种都会将结合降低 5 个数量级以上。由于 HCF-1 多肽与 OGT 结合如此紧密,因此我们测试了其抑制酶功能的能力。我们发现 HCF3R 在体外和细胞中均有效抑制 OGT,并利用这一发现开发了一种基因编码的诱导型 OGT 抑制剂,该抑制剂可以用小分子降解,从而对 OGT 进行可逆和可调的抑制。
更新日期:2024-09-14
中文翻译:

鉴定具有 Picomolar 亲和力的 O-GlcNAc 转移酶多肽抑制剂
O-GlcNAc 转移酶 (OGT) 是一种必需的哺乳动物酶,可结合数千种不同的蛋白质,包括它糖基化的底物和调节其生物学的非底物相互作用物。OGT 还具有一种蛋白水解底物,即转录共调节宿主细胞因子 1 (HCF-1),它在谷氨酸侧链糖基化引发的一系列中央重复序列中裂解。尽管 HCF-1 是 OGT 最突出的结合伴侣,但它对酶的亲和力尚未被量化。在这里,我们报道了一种时间分辨的 Förster 共振能量转移测定法,用于测量配体与 OGT 的结合,并表明 HCF-1 衍生的多肽 (HCF3R) 与酶 (KD≤ 85 pM) 以皮摩尔亲和力结合。这种高亲和力在很大程度上是由 OGT 四肽重复结构域中的保守天冬酰胺驱动的,这些天冬酰胺与 HCF-1 肽骨架形成双齿接触;用丙氨酸替换这些天冬酰胺中的任何一种都会将结合降低 5 个数量级以上。由于 HCF-1 多肽与 OGT 结合如此紧密,因此我们测试了其抑制酶功能的能力。我们发现 HCF3R 在体外和细胞中均有效抑制 OGT,并利用这一发现开发了一种基因编码的诱导型 OGT 抑制剂,该抑制剂可以用小分子降解,从而对 OGT 进行可逆和可调的抑制。































 京公网安备 11010802027423号
京公网安备 11010802027423号