当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid and Highly Selective Fe(IV) Generation by Fe(II)-Peroxyacid Advanced Oxidation Processes: Mechanistic Investigation via Kinetics and Density Functional Theory
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acs.est.4c05234 Junyue Wang 1 , Juhee Kim 1 , Jiaqi Li 1 , Caroline Krall 1 , Virender K Sharma 2 , Daniel C Ashley 3 , Ching-Hua Huang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acs.est.4c05234 Junyue Wang 1 , Juhee Kim 1 , Jiaqi Li 1 , Caroline Krall 1 , Virender K Sharma 2 , Daniel C Ashley 3 , Ching-Hua Huang 1
Affiliation
|
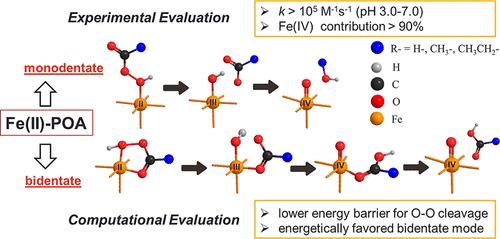
High-valent iron (Fe(IV/V/VI)) has been widely applied in water decontamination. However, common Fe(II)-activating oxidants including hydrogen peroxide (H2O2) and persulfate react slowly with Fe(II) and exhibit low selectivity for Fe(IV) production due to the cogeneration of radicals. Herein, we report peroxyacids (POAs; R–C(O)OOH) that can react with Fe(II) more than 3 orders of magnitude faster than H2O2, with high selectivity for Fe(IV) generation. Rapid degradation of bisphenol A (BPA, an endocrine disruptor) was achieved by the combination of Fe(II) with performic acid (PFA), peracetic acid (PAA), or perpropionic acid (PPA) within one second. Experiments with phenyl methyl sulfoxide (PMSO) and tert-butyl alcohol (TBA) revealed Fe(IV) as the major reactive species in all three Fe(II)-POA systems, with a minor contribution of radicals (i.e., •OH and R–C(O)O•). To understand the exceptionally high reactivity of POAs, a detailed computational comparison among the Fenton-like reactions with step-by-step thermodynamic evaluation was conducted. The high reactivity is attributed to the lower energy barriers for O–O bond cleavage, which is determined as the rate-limiting step for the Fenton-like reactions, and the thermodynamically favorable bidentate binding pathway of POA with iron. Overall, this study advances knowledge on POAs as novel Fenton-like reagents and sheds light on computational chemistry for these systems.
中文翻译:

Fe(II)-过氧酸高级氧化过程快速、高选择性生成 Fe(IV):通过动力学和密度泛函理论进行机理研究
高价铁(Fe(IV/V/VI))已广泛应用于水净化。然而,常见的Fe(II)活化氧化剂,包括过氧化氢(H 2 O 2 )和过硫酸盐,与Fe(II)反应缓慢,并且由于自由基的共生,对Fe(IV)生产的选择性较低。在此,我们报道了过氧酸(POAs;R–C(O)OOH),它与 Fe(II) 的反应速度比 H 2 O 2快 3 个数量级,并且对 Fe(IV) 的生成具有高选择性。通过 Fe(II) 与过甲酸 (PFA)、过乙酸 (PAA) 或过丙酸 (PPA) 的结合在一秒钟内实现双酚 A(BPA,一种内分泌干扰物)的快速降解。使用苯甲基亚砜 (PMSO) 和叔丁醇 (TBA) 进行的实验表明,Fe(IV) 是所有三种 Fe(II)-POA 体系中的主要活性物质,自由基的贡献较小(即, • OH 和 R) –C(O)O • )。为了了解 POAs 极高的反应活性,我们对类芬顿反应进行了详细的计算比较,并进行了逐步的热力学评估。高反应活性归因于O-O键断裂的较低能垒(被确定为类芬顿反应的限速步骤)以及POA与铁的热力学有利的双齿结合途径。总体而言,这项研究增进了对 POA 作为新型类芬顿试剂的认识,并为这些系统的计算化学提供了线索。
更新日期:2024-09-14
中文翻译:

Fe(II)-过氧酸高级氧化过程快速、高选择性生成 Fe(IV):通过动力学和密度泛函理论进行机理研究
高价铁(Fe(IV/V/VI))已广泛应用于水净化。然而,常见的Fe(II)活化氧化剂,包括过氧化氢(H 2 O 2 )和过硫酸盐,与Fe(II)反应缓慢,并且由于自由基的共生,对Fe(IV)生产的选择性较低。在此,我们报道了过氧酸(POAs;R–C(O)OOH),它与 Fe(II) 的反应速度比 H 2 O 2快 3 个数量级,并且对 Fe(IV) 的生成具有高选择性。通过 Fe(II) 与过甲酸 (PFA)、过乙酸 (PAA) 或过丙酸 (PPA) 的结合在一秒钟内实现双酚 A(BPA,一种内分泌干扰物)的快速降解。使用苯甲基亚砜 (PMSO) 和叔丁醇 (TBA) 进行的实验表明,Fe(IV) 是所有三种 Fe(II)-POA 体系中的主要活性物质,自由基的贡献较小(即, • OH 和 R) –C(O)O • )。为了了解 POAs 极高的反应活性,我们对类芬顿反应进行了详细的计算比较,并进行了逐步的热力学评估。高反应活性归因于O-O键断裂的较低能垒(被确定为类芬顿反应的限速步骤)以及POA与铁的热力学有利的双齿结合途径。总体而言,这项研究增进了对 POA 作为新型类芬顿试剂的认识,并为这些系统的计算化学提供了线索。































 京公网安备 11010802027423号
京公网安备 11010802027423号