当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overlooked Complexation and Competition Effects of Phenolic Contaminants in a Mn(II)/Nitrilotriacetic Acid/Peroxymonosulfate System: Inhibited Generation of Primary and Secondary High-Valent Manganese Species
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acs.est.4c07370 Hongyu Zhou 1 , Shuang Zhong 1 , Junwen Chen 1 , Shiying Ren 1 , Wei Ren 1 , Bo Lai 2 , Xiaohong Guan 3 , Tianyi Ma 4 , Shaobin Wang 1 , Xiaoguang Duan 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-14 , DOI: 10.1021/acs.est.4c07370 Hongyu Zhou 1 , Shuang Zhong 1 , Junwen Chen 1 , Shiying Ren 1 , Wei Ren 1 , Bo Lai 2 , Xiaohong Guan 3 , Tianyi Ma 4 , Shaobin Wang 1 , Xiaoguang Duan 1
Affiliation
|
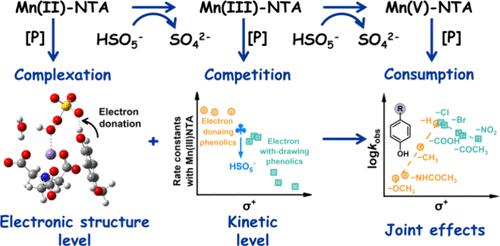
Organic contaminants with lower Hammett constants are typically more prone to being attacked by reactive oxygen species (ROS) in advanced oxidation processes (AOPs). However, the interactions of an organic contaminant with catalytic centers and participating ROS are complex and lack an in-depth understanding. In this work, we observed an abnormal phenomenon in AOPs that the degradation of electron-rich phenolics, such as 4-methoxyphenol, acetaminophen, and 4-presol, was unexpectedly slower than electron-deficient phenolics in a Mn(II)/nitrilotriacetic acid/peroxymonosulfate (Mn(II)/NTA/PMS) system. The established quantitative structure–activity relationship revealed a volcano-type dependence of the degradation rates on the Hammett constants of pollutants. Leveraging substantial analytical techniques and modeling analysis, we concluded that the electron-rich phenolics would inhibit the generation of both primary (Mn(III)NTA) and secondary (Mn(V)NTA) high-valent manganese species through complexation and competition effects. Specifically, the electron-rich phenolics would form a hydrogen bond with Mn(II)/NTA/PMS through outer-sphere interactions, thereby reducing the electrophilic reactivity of PMS to accept the electron transfer from Mn(II)NTA, and slowing down the generation of reactive Mn(III)NTA. Furthermore, the generated Mn(III)NTA is more inclined to react with electron-rich phenolics than PMS due to their higher reaction rate constants (8314 ± 440, 6372 ± 146, and 6919 ± 31 M–1 s–1 for 4-methoxyphenol, acetaminophen, and 4-presol, respectively, as compared with 671 M–1 s–1 for PMS). Consequently, the two-stage inhibition impeded the generation of Mn(V)NTA. In contrast, the complexation and competition effects are insignificant for electron-deficient phenolics, leading to declined reaction rates when the Hammett constants of pollutants increase. For practical applications, such complexation and competition effects would cause the degradation of electron-rich phenolics to be more susceptible to water matrixes, whereas the degradation of electron-deficient phenolics remains largely unaffected. Overall, this study elucidated the intricate interaction mechanisms between contaminants and reactive metal species at both the electronic and kinetic levels, further illuminating their implications for practical treatment.
中文翻译:

被忽视的酚类污染物在 Mn(II)/次氮基三乙酸/过氧一硫酸盐系统中的络合和竞争效应:抑制初级和次级高价锰种类的生成
在高级氧化过程 (AOP) 中,具有较低哈米特常数的有机污染物通常更容易受到活性氧 (ROS) 的侵蚀。然而,有机污染物与催化中心和参与的 ROS 之间的相互作用很复杂,缺乏深入的理解。在这项工作中,我们在 AOPs 中观察到一个异常现象,即富电子酚类物质(如 4-甲氧基苯酚、对乙酰氨基酚和 4-presol)的降解出乎意料地慢于 Mn(II)/次氮基三乙酸/过氧一硫酸盐 (Mn(II)/NTA/PMS) 系统中的缺电子酚类物质。已建立的定量构效关系揭示了降解速率对污染物哈米特常数的火山型依赖性。利用大量的分析技术和建模分析,我们得出结论,富电子酚类物质会通过络合和竞争效应抑制初级 (Mn(III)NTA) 和次级 (Mn(V)NTA) 高价锰种类的产生。具体来说,富含电子的酚类物质会通过外球相互作用与 Mn(II)/NTA/PMS 形成氢键,从而降低 PMS 接受来自 Mn(II)NTA 的电子转移的亲电反应性,并减慢反应性 Mn(III)NTA 的产生。此外,生成的 Mn(III)NTA 比 PMS 更倾向于与富含电子的酚类物质反应,因为它们的反应速率常数更高(4-甲氧基苯酚、对乙酰氨基酚和 4-presol 分别为 8314 ± 440、6372 ± 146 和 6919 ±分别为 31 M-1 s-1,而 PMS 为 671 M-1 s-1)。因此,两阶段抑制阻碍了 Mn(V)NTA 的产生。 相比之下,对于缺电子酚类物质来说,络合和竞争效应微不足道,当污染物的哈米特常数增加时,会导致反应速率下降。对于实际应用,这种络合和竞争效应将导致富电子酚类化合物的降解更容易受到水基质的影响,而缺电子酚类化合物的降解在很大程度上不受影响。总体而言,本研究在电子和动力学水平上阐明了污染物和活性金属种类之间错综复杂的相互作用机制,进一步阐明了它们对实际处理的影响。
更新日期:2024-09-14
中文翻译:

被忽视的酚类污染物在 Mn(II)/次氮基三乙酸/过氧一硫酸盐系统中的络合和竞争效应:抑制初级和次级高价锰种类的生成
在高级氧化过程 (AOP) 中,具有较低哈米特常数的有机污染物通常更容易受到活性氧 (ROS) 的侵蚀。然而,有机污染物与催化中心和参与的 ROS 之间的相互作用很复杂,缺乏深入的理解。在这项工作中,我们在 AOPs 中观察到一个异常现象,即富电子酚类物质(如 4-甲氧基苯酚、对乙酰氨基酚和 4-presol)的降解出乎意料地慢于 Mn(II)/次氮基三乙酸/过氧一硫酸盐 (Mn(II)/NTA/PMS) 系统中的缺电子酚类物质。已建立的定量构效关系揭示了降解速率对污染物哈米特常数的火山型依赖性。利用大量的分析技术和建模分析,我们得出结论,富电子酚类物质会通过络合和竞争效应抑制初级 (Mn(III)NTA) 和次级 (Mn(V)NTA) 高价锰种类的产生。具体来说,富含电子的酚类物质会通过外球相互作用与 Mn(II)/NTA/PMS 形成氢键,从而降低 PMS 接受来自 Mn(II)NTA 的电子转移的亲电反应性,并减慢反应性 Mn(III)NTA 的产生。此外,生成的 Mn(III)NTA 比 PMS 更倾向于与富含电子的酚类物质反应,因为它们的反应速率常数更高(4-甲氧基苯酚、对乙酰氨基酚和 4-presol 分别为 8314 ± 440、6372 ± 146 和 6919 ±分别为 31 M-1 s-1,而 PMS 为 671 M-1 s-1)。因此,两阶段抑制阻碍了 Mn(V)NTA 的产生。 相比之下,对于缺电子酚类物质来说,络合和竞争效应微不足道,当污染物的哈米特常数增加时,会导致反应速率下降。对于实际应用,这种络合和竞争效应将导致富电子酚类化合物的降解更容易受到水基质的影响,而缺电子酚类化合物的降解在很大程度上不受影响。总体而言,本研究在电子和动力学水平上阐明了污染物和活性金属种类之间错综复杂的相互作用机制,进一步阐明了它们对实际处理的影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号