当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of Thieno[3, 2-b]pyridinone derivatives exhibiting potent activities against Mycobacterium tuberculosis in vivo by targeting Enoyl-ACP reductase
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-08-31 , DOI: 10.1016/j.ejmech.2024.116806 Lihong Liang , Zhiyong Liu , Jie Chen , Qin Zha , Yihuan Zhou , Jun Li , Yangbo Hu , Xinwen Chen , Tianyu Zhang , Niuniu Zhang
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-08-31 , DOI: 10.1016/j.ejmech.2024.116806 Lihong Liang , Zhiyong Liu , Jie Chen , Qin Zha , Yihuan Zhou , Jun Li , Yangbo Hu , Xinwen Chen , Tianyu Zhang , Niuniu Zhang
|
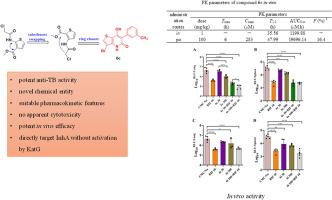
In this study, a series of novel thieno [3, 2-b ]pyridinone derivatives were designed and synthesized using a scaffold hopping strategy. Six compounds showed potent anti-mycobacterial activity (minimum inhibitory concentration (MIC) ≤ 1 μg/mL) against Mycobacterium tuberculosis (Mtb) UAlRa. Compound 6c displayed good activity against Mtb UAlRv (MIC = 0.5–1 μg/mL). Compounds 6c and 6i also showed activity against Mtb UAlRa in macrophages and exhibited low cytotoxicity against LO-2 cells. The selected compounds displayed a narrow antibacterial spectrum, with no activity against representative Gram-positive, Gram-negative bacteria, as well as fungi. Furthermore, compound 6c demonstrated favorable oral pharmacokinetic properties with a T 1/2 value of 47.99 h and exhibited good in vivo activity in an acute mouse model of tuberculosis (TB). The target of compound 6c was identified as a NADH-dependent enoyl-acyl carrier protein reductase (InhA) by genome sequencing of spontaneously compound 6c -resistant Mtb mutants, indicating that compound 6c may not require activation and can directly target InhA. In vitro antimicrobial assays against a recombinant M. smegmatis overexpressing the Mtb-InhA, along with InhA inhibition assays, confirmed that InhA is the target of thieno [3, 2-b ]pyridinone derivatives. Overall, this study identified thieno [3, 2-b ]pyridinone scaffold as a novel chemotype that is promising for the development of anti-TB agents.
中文翻译:

通过靶向烯酰-ACP 还原酶在体内表现出对结核分枝杆菌有效活性的噻吩并[3,2-b]吡啶酮衍生物的设计与合成
在本研究中,使用支架跳跃策略设计并合成了一系列新型噻吩基 [3, 2-b] 吡啶酮衍生物。6 种化合物对结核分枝杆菌 (Mtb) UAlRa 显示出有效的抗分枝杆菌活性 (最低抑菌浓度 ≤ 1 μg/mL)。化合物 6c 对 Mtb UAlRv 表现出良好的活性 (MIC = 0.5–1 μg/mL)。化合物 6c 和 6i 在巨噬细胞中也显示出对 Mtb UAlRa 的活性,并且对 LO-2 细胞表现出低细胞毒性。所选化合物显示出较窄的抗菌谱,对代表性革兰氏阳性、革兰氏阴性细菌以及真菌没有活性。此外,化合物 6c 表现出良好的口服药代动力学特性,T1/2 值为 47.99 h,并且在急性结核病 (TB) 小鼠模型中表现出良好的体内活性。通过对自发化合物 6c 耐药的 Mtb 突变体进行基因组测序,将化合物 6c 的靶点鉴定为 NADH 依赖性烯酰酰基载体蛋白还原酶 (InhA),表明化合物 6c 可能不需要激活,可以直接靶向 InhA。针对过表达 Mtb-InhA 的重组耻垢分枝杆菌的体外抗菌测定以及 InhA 抑制测定证实,InhA 是噻吩并 [3, 2-b] 吡啶酮衍生物的靶标。总体而言,本研究确定噻吩并 [3, 2-b] 吡啶酮支架是一种新型化学型,有望开发抗结核药物。
更新日期:2024-08-31
中文翻译:

通过靶向烯酰-ACP 还原酶在体内表现出对结核分枝杆菌有效活性的噻吩并[3,2-b]吡啶酮衍生物的设计与合成
在本研究中,使用支架跳跃策略设计并合成了一系列新型噻吩基 [3, 2-b] 吡啶酮衍生物。6 种化合物对结核分枝杆菌 (Mtb) UAlRa 显示出有效的抗分枝杆菌活性 (最低抑菌浓度 ≤ 1 μg/mL)。化合物 6c 对 Mtb UAlRv 表现出良好的活性 (MIC = 0.5–1 μg/mL)。化合物 6c 和 6i 在巨噬细胞中也显示出对 Mtb UAlRa 的活性,并且对 LO-2 细胞表现出低细胞毒性。所选化合物显示出较窄的抗菌谱,对代表性革兰氏阳性、革兰氏阴性细菌以及真菌没有活性。此外,化合物 6c 表现出良好的口服药代动力学特性,T1/2 值为 47.99 h,并且在急性结核病 (TB) 小鼠模型中表现出良好的体内活性。通过对自发化合物 6c 耐药的 Mtb 突变体进行基因组测序,将化合物 6c 的靶点鉴定为 NADH 依赖性烯酰酰基载体蛋白还原酶 (InhA),表明化合物 6c 可能不需要激活,可以直接靶向 InhA。针对过表达 Mtb-InhA 的重组耻垢分枝杆菌的体外抗菌测定以及 InhA 抑制测定证实,InhA 是噻吩并 [3, 2-b] 吡啶酮衍生物的靶标。总体而言,本研究确定噻吩并 [3, 2-b] 吡啶酮支架是一种新型化学型,有望开发抗结核药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号