当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Replacement of the essential nitro group by electrophilic warheads towards nitro-free antimycobacterial benzothiazinones
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116849 Héctor Torres-Gómez 1 , François Keiff 1 , Peter Hortschansky 2 , Freddy Bernal 1 , Valerie Kerndl 1 , Florian Meyer 1 , Nina Messerschmidt 1 , Michael Dal Molin 3 , Thomas Krüger 2 , Jan Rybniker 4 , Axel A Brakhage 5 , Florian Kloss 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116849 Héctor Torres-Gómez 1 , François Keiff 1 , Peter Hortschansky 2 , Freddy Bernal 1 , Valerie Kerndl 1 , Florian Meyer 1 , Nina Messerschmidt 1 , Michael Dal Molin 3 , Thomas Krüger 2 , Jan Rybniker 4 , Axel A Brakhage 5 , Florian Kloss 1
Affiliation
|
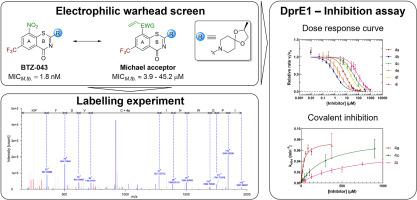
Nitrobenzothiazinones (BTZs) are undergoing late-stage development as a novel class of potent antitubercular drug candidates with two compounds in clinical phases. BTZs inhibit decaprenylphosphoryl-β-d -ribose oxidase 1 (DprE1), a key enzyme in cell wall biosynthesis of mycobacteria. Their mechanism of action involves an in-situ -reduction of the nitro moiety to a reactive nitroso intermediate capable of covalent binding to Cys387 in the catalytic cavity. The electron-deficient nature of the aromatic core is a key driver for the formation of hydride-Meisenheimer complexes (HMC) as main metabolites in vivo . To mimic the electrophilic character of the nitroso moiety, bioisosteric replacement with different electrophilic warheads was attempted to reduce HMC formation without compromising covalent reactivity. Herein, we synthesized and characterized various covalent warheads covering different reaction principles. Covalent inhibition was confirmed for most active antimycobacterial compounds by enzymatic inhibition assays and peptide fragment analysis.
中文翻译:

用亲电弹头取代必需的硝基,转为无硝基抗分枝杆菌苯并噻嗪酮
硝基苯并噻嗪酮 (BTZ) 作为一类新型强效抗结核候选药物正在进行后期开发,其中两种化合物处于临床阶段。BTZ 抑制十碳烯基磷酸基-β-d-核糖氧化酶 1 (DprE1),分枝杆菌细胞壁生物合成中的关键酶。它们的作用机制包括将硝基部分原位还原为能够在催化腔中与 Cys387 共价结合的反应性亚硝基中间体。芳香族核心的缺电子性质是形成氢化物-迈森海默复合物 (HMC) 作为体内主要代谢物的关键驱动因素。为了模拟亚硝基部分的亲电特性,尝试用不同的亲电弹头进行生物等位置换,以减少 HMC 的形成,而不会影响共价反应性。在此,我们合成并表征了涵盖不同反应原理的各种共价弹头。通过酶抑制测定和肽片段分析证实大多数活性抗分枝杆菌化合物具有共价抑制作用。
更新日期:2024-09-05
中文翻译:

用亲电弹头取代必需的硝基,转为无硝基抗分枝杆菌苯并噻嗪酮
硝基苯并噻嗪酮 (BTZ) 作为一类新型强效抗结核候选药物正在进行后期开发,其中两种化合物处于临床阶段。BTZ 抑制十碳烯基磷酸基-β-d-核糖氧化酶 1 (DprE1),分枝杆菌细胞壁生物合成中的关键酶。它们的作用机制包括将硝基部分原位还原为能够在催化腔中与 Cys387 共价结合的反应性亚硝基中间体。芳香族核心的缺电子性质是形成氢化物-迈森海默复合物 (HMC) 作为体内主要代谢物的关键驱动因素。为了模拟亚硝基部分的亲电特性,尝试用不同的亲电弹头进行生物等位置换,以减少 HMC 的形成,而不会影响共价反应性。在此,我们合成并表征了涵盖不同反应原理的各种共价弹头。通过酶抑制测定和肽片段分析证实大多数活性抗分枝杆菌化合物具有共价抑制作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号